The chemical structure of silicone resins and silicone combination resins results in outstanding properties almost impossible to achieve with other products. These resins are used in numerous industrial applications, particularly as binders for formulating coatings. Applications range from impregnations and weather-resistant exterior coatings for building conservation to high-temperature-resistant coatings. Silicone resins are used particularly in the latter due to their higher silicone content. The resulting decrease in organic content translates into a greater ability to withstand higher temperatures than silicone hybrid resins traditionally offer.
High-temperature-resistant coatings, which are often used for exhausts, stacks, industrial furnaces, barbecues and combustion chambers, must meet high demands in addition to thermal stability. The properties listed below make silicone resins ideally suited to this area:
- Thermal stability;
- Weathering resistance;
- Retention of elasticity even at low temperatures;
- Chemical resistance to aromatic and aliphatic solvents;
- Low surface tension;
- Water repellency, surface activity;
- Release and surface slip properties.
The silicone resins used as binders in the form of solutions, liquid resins and aqueous emulsions in the field of high-temperature-resistant coatings are comprised mainly of methyl silicone resins and methyl-phenyl silicone resins. Silicone resins that contain only phenyl groups are very limited in their application as they have the particular disadvantage of long-lasting thermoplasticity.1
Silicone Resin Properties
Methyl silicone resins are the polymethylsiloxanes with the lowest carbon content. The chemical analogy to silica determines the partially inorganic character of this group of resins. This explains properties such as the relatively high hardness, low thermoplasticity, poor pigment affinity, particular affinity to inorganic mineral products, and incompatibility with organic resins. The methyl silicone resins exhibit excellent water repellency even in a solvent-free, partially crosslinked state at room temperature. Methyl silicone resins are marketed commercially mainly in aromatic or aliphatic solvents or alcohols but are also available as 100%-liquid resins.
Besides methyl groups, methyl-phenyl silicone resins also have a phenyl group content that is normally over 20%.2 The phenyl groups in the methyl-phenyl silicone resins significantly improve compatibility with other organic binders. Furthermore, the phenyl-methyl silicone resins exhibit better pigment affinity and increased thermoplasticity. Compared to methyl silicone resins, methyl-phenyl silicone resins often require less substrate pretreatment. Due to the improved compatibility with other organic resins, methyl-phenyl silicone resins are frequently used for the synthesis of silicone combination resins. Suitable solvents for methyl-phenyl silicone resins are primarily aromatics and esters.
Silicone resins for high-temperature-resistant coatings can only be used in pigmented formulations. Long-term heat resistance is strongly dependent on the formulation and particularly on the choice of pigment. Long-term heat resistance of approx. 650 °C can be achieved with some inorganic pigments. Long-term exposure to higher temperatures eventually results in complete oxidation of the organic groups.
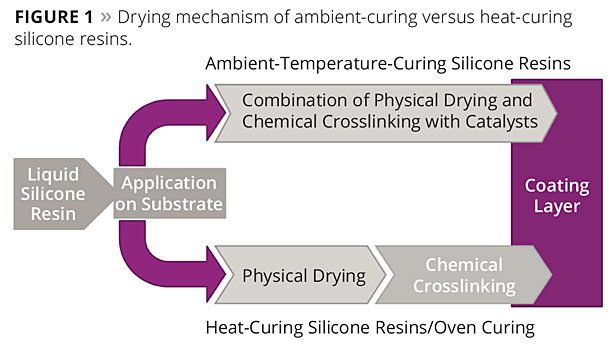
Oven-Curing and Ambient-Curing Systems
In principle, both the methyl-phenyl silicone resins and the methyl silicone resins can be divided into two different types: the classic oven-curing systems, which are dried at high temperatures in ovens (such as -SILIKOPHEN®P resins), and the newer, versatile, ambient-curing systems (SILIKOPHEN®AC resins).
For oven-curing systems, physical drying takes place first, i.e., solvents volatilize from the coatings, then chemical crosslinking is subsequently initiated by baking at high temperatures.
In contrast, ambient-curing systems require no heat input. Physical drying and chemical crosslinking take place simultaneously at room temperature. The chemical crosslinking is initiated not by the application of heat but by atmospheric moisture in the presence of catalysts. The different curing conditions and processes are shown in Figure 1.
Suitable catalysts to accelerate ambient curing systems in the presence of atmospheric moisture are tetra-n-butyl titanate (TnBT) or mixtures of TnBT and tetramethylguan-idine (TMG). In such combinations the TnBT reacts as a Lewis acid by forming chemical bonds with the polymer. On the other hand, TMG reacts as a strong base, accelerating the reaction. Both catalysts are soluble and miscible in xylene. Atmospheric moisture is an important factor, and is required to hydrolyze the alkoxy groups of ambient-curing silicone resins. Ultimately, condensation of the silanol groups formed by this reaction occurs during curing of the film.
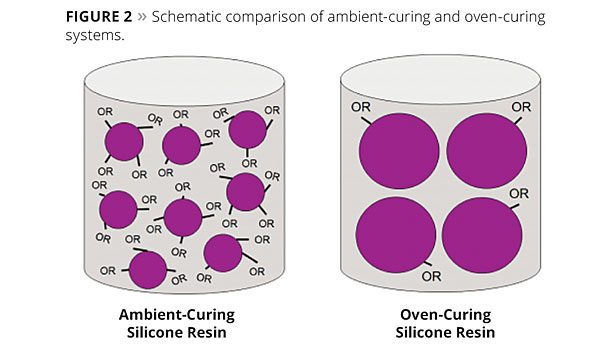
Structural Difference Between the Systems
As shown in Figure 2, the decisive structural difference between ambient- and oven-curing systems is the density of functional groups and molecular weight of the silicone resins. The particle size symbolizes the molecular weight of the silicone resins, while the OR groups represent the alkoxy groups. Their number per particle is a measure of the density of functional groups.
The methyl silicone resin and methyl-phenyl silicone resin oven-curing systems, which cure at high temperatures, have significantly higher molecular weight than ambient-curing resins and a very low density of functional groups with regard to alkoxy and silanol groups. Such silicone resins are typically stoved for 30 min at approximately 250 °C to achieve a fully cured and hard coating.
The ambient-curing resins are high alkoxy-functional, low-molecular-weight silicone resin building blocks. The low molecular weight results in products with very low viscosities and thus very good processability. Moreover, these systems have a high active matter content, which enables the formulation of high-solids coatings systems with very low VOC content.
As a rule, the alkoxy content of ambient-curing silicone resins is approximately 30% w/w, and they are available commercially with solids content of up to 100%. In the field of methyl-phenyl silicone resins, the latest developments from Evonik in catalysis of the hydrolysis/condensation reaction have, for the first time, enabled industrial-scale curing at room temperature. The advantages resulting from curing at room temperature are obvious. Oven curing at high temperatures limits the size of the items to be coated to the dimensions of the available oven. In the ambient temperature curing process, large items can be coated with silicone resins in the open air, which opens up new fields of application for high-temperature-resistant coatings. The use of ambient-curing silicone resins instead of oven curing at high temperatures can also markedly reduce energy consumption.
Conclusion
The commercial success of silicone resins in the field of high-temperature-resistant coatings stems from their unique properties. Both the classic oven-curing systems and ambient-curing systems are very successful. Curing at room temperature is achieved in the latter by atmospheric moisture in the presence of catalysts, thus saving the energy necessary for stoving. The size of items to be coated is not limited by the size of the oven, thus opening up further areas of application, particularly in industrial applications.
References
1 Heilen. Silicone resins and their combinations, Vincentz Network, Hannover, 2005.
2 Stoye/Freitag, Lackharze, Carl Hanser Verlag. München - Wien, 1996, pp. 343.
For more information, visit www.tego.us.
By Sascha Herrwerth, Ph.D., Director Technical Service Specialty Resins; Annika König, Ph.D., Product Management Specialty Resins; and Marion Siemens, Senior Technical Service Manager Specialty Resins | Evonik Industries, AG, Essen, Germany





Report Abusive Comment