This article addresses the strong market demand for pigment-stabilizing oligomers to obtain 100%-solids, VOC-free formulations. A new family of dendritic acrylate oligomers with different functionalities that address various stabilization mechanisms (pigment wetting, electrostatic and steric stabilization) will be discussed. Inks and coatings based on these oligomers are very fast curing and prone to substantially less oxygen inhibition. They demonstrate good adhesion to different substrates and possess excellent chemical and scratch resistance.
Introduction
There are three main stages for dispersing pigments in paint, ink or coating solutions -wetting, grinding and stabilization of the pigment suspension. During the initial pigment wetting stage, the moisture on the surface of the pigment and air trapped within the pigment agglomerates. The extent of wetting depends on the viscosity of the solution and the surface tension of the pigments and dispersing media. In the grinding stage, pigment agglomerates are divided into smaller pieces and distributed uniformly. Dispersing agents that can adsorb onto the pigment surface are often used to avoid formation of flocculates and hence stabilize pigment dispersions. Electrostatic and steric stabilization are the two principal mechanisms for pigment stabilization. Polyphosphate and polycarboxylic acid derivatives are usually used as polyelectrolytes to provide electrostatic stabilization. In a solution with low dielectric constant, steric stabilization is the main type of stabilization. Additive or binder chains should be adsorped onto the surface of the pigments, so as not to allow flocculation of particles for effective steric stabilization.1
Dendritic oligomers are highly branched sterically hindered oligomers. Various chemistries including polyurethane (meth)acrylates,2 aminoacrylates3 and thioacrylates4 are reported in literature. Of these, thioacrylate dendritic oligomers are of special interest to us due to their exceptional mechanical and physical properties in hard, protective coatings. They exhibit very good chemical and high-temperature resistance, as well as excellent adhesion properties to metal, glass and a variety of plastics. This article focuses on the pigment stabilization properties of the new dendritic thioacrylate oligomers.
Experimental
All oligomers were analyzed with gel permeation chromatography and FTIR. Viscosities were measured using a Brookfield CAP 2000+ viscometer with CAP 2 Spindle. 3% photoinitiator was added to all oligomers and mixed until fully dissolved. A 12.7-micron wet coating of this mixture was applied to glass for the cure speed analysis. For all other physical property testing the oligomer/photoinitiator mixture was diluted in acetone to the level of 40% solids. A 12.7-micron wet coating was then applied to the substrates so that a thickness of 5 microns would be achieved after the solvent evaporated.
All coatings were cured using a Fusion 300 processor with an H-bulb. Single passes under the lamp at a conveyor belt speed of 5 and 20 ft/min were performed for the cure speed analysis. For all other tests, coatings were cured at a belt speed of 5 ft/min alone. The cured coatings were allowed to stand at room temperature for two hours before testing. The rate of acrylate conversion was determined by analyzing the FTIR of the oligomer coatings before and after cure. The relative difference in the acrylate peak height at ~ 810 cm-1 and ~ 1630 cm-1 was then calculated as a percentage of the initial peak height in the uncured oligomer.
Pencil hardness of the cured coatings was determined in accordance with ASTM Method D3363. Scratch resistance was determined by comparing the % haze of the coating before and after being abraded with steel wool for five passes. % haze was measured using a colorimetric spectrophotometer.
Chemical resistance to solvent was determined in a manner based on ASTM Method D5402 using methyl ethyl ketone (MEK) as solvent. A maximum of 200 solvent double-rubs were performed. Chemical resistance to 70% nitric acid was determined in a manner based on ASTM Method D1308 on a glass substrate. The test areas were covered and the materials allowed to sit until coating integrity was compromised.
Coating surface tension was determined using AccuDyne Test marker pens with the result being the lowest marker that did not bead up on the coating. Adhesion to various substrates was tested in accordance with ASTM Method B2197.
Results and Discussion
As detailed below, seven hyperbranched dendritic acrylate oligomers were synthesized and tested in order to investigate the effect of various structural changes on coating physical properties and pigment stabilization ability. A polyester acrylate oligomer (A), a urethane acrylate oligomer (B) and a melamine acrylate oligomer (C) were also tested as comparative controls. Such oligomers are typically used in pigmented systems.
Oligomer Structure and Properties
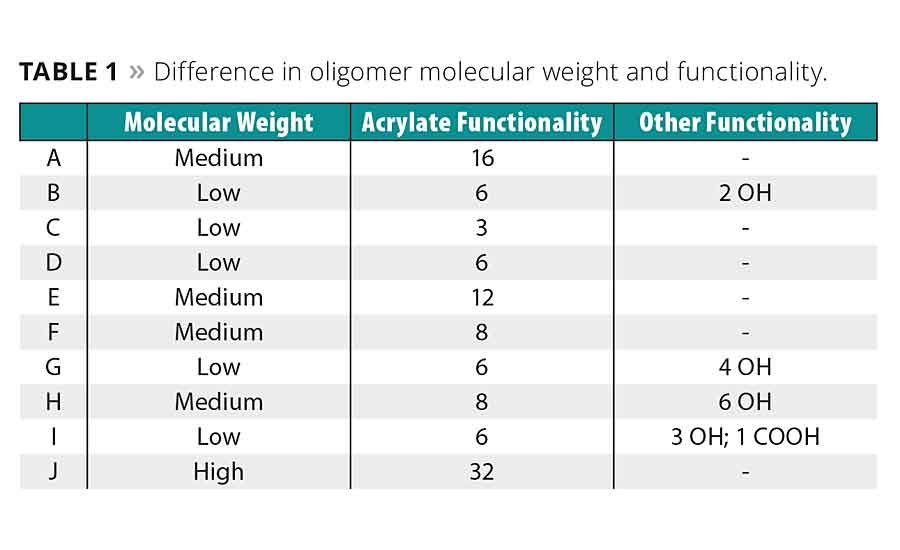
The idealized structure of oligomers D-J is shown in Figure 1. The oligomers primarily differ in terms of molecular weight and acrylate functionality. However, oligomers G and H have added hydroxyl functionality, and oligomer I has both hydroxyl and carboxylic acid functionality. Table 1 further elaborates on these structures by noting these differences.
Oligomer Liquid Viscosity
Liquid viscosity is an important property, as it can have a marked impact on formulation possibilities and processing. The viscosity data for each oligomer is shown in Table 2.
As expected, oligomer viscosity is largely controlled by molecular weight and intermolecular bonding (i.e., hydrogen bonding). The urethane containing B and the highest-molecular-weight, highest-functionality dendritic J had the highest viscosity. The hydroxyl and/or acid groups of G-I also resulted in an increased viscosity when compared to D-F, which do not have those functional groups. Despite the differences, though, all the oligomers can be diluted very well in solvent or monomer to whatever viscosity the application might require.
Cure Speed
The percentage of acrylates converted upon cure at a belt speed of 5 ft/min and 20 ft/min is shown in Figure 2. All the oligomer coatings showed a good cure response. The percentage of acrylates converted at 20 ft/min was very close to the percentage converted at 5 ft/min, even though the film was exposed to four times the energy at the slower belt speed.
Overall, the percent of acrylate conversion appears linked to molecular mobility before and during cure. Oligomers B, I and J have the highest liquid viscosity but lowest percent conversion due to the decreased mobility at those viscosities. Conversely, the low-viscosity polyester acrylate, A, had one of the highest conversions.
Functionality also plays a role, as illustrated in the comparisons between D-E and G-H, with the higher functionality, in general, resulting in faster cure. However, this likely is limited by the fact that molecular mobility will decrease with increasing conversion. At a certain point of conversion, any remaining unconverted acrylates will simply be unable to move and react. This is one explanation for the trifunctional melamine acrylate, C, having such a high conversion percentage where J, with 32 acrylates, is so low.
Oxygen Inhibition
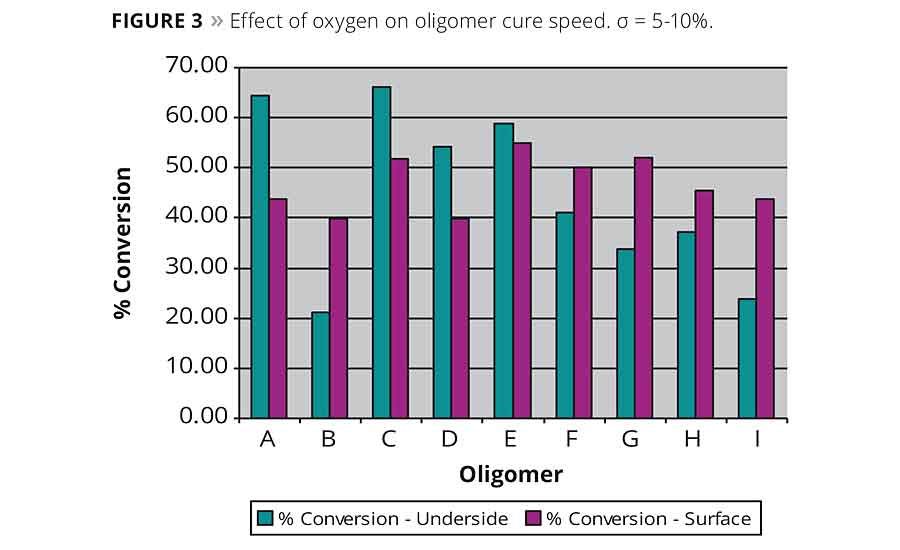
It is well known that the presence of oxygen has a negative effect on photopolymerization. Typically, the effect is more pronounced at a coating’s surface where it is in contact with the atmosphere. Formulation or process modifications are normally required to overcome this oxygen inhibition layer and achieve full cure.
Figure 3 shows the percent conversion of acrylate groups at the surface and on the underside of the oligomer coatings when cured at a belt speed of 5 ft/min. All the coatings based on the new dendritic oligomers except D and possibly E show no significant difference between the percent conversion at the surface compared to the underside. In fact, in two cases, namely with G and I, the results suggest that the surface actually cured better. The oxygen inhibition effect was most pronounced with the standard oligomer A.
Hardness and Scratch Resistance
Coating hardness and resistance to scratching are important physical properties to consider in any application. As shown in Table 3, all oligomers have good pencil hardness of 5H or above. A majority of the new dendritic oligomers, F-J, have exceptional scratch resistance as well. Coatings based on these oligomers showed very little change in clarity after being abraded with steel wool. Although D and E showed a slight increase in susceptibility to scratching, both were much better than the standards A and C.
Chemical Resistance
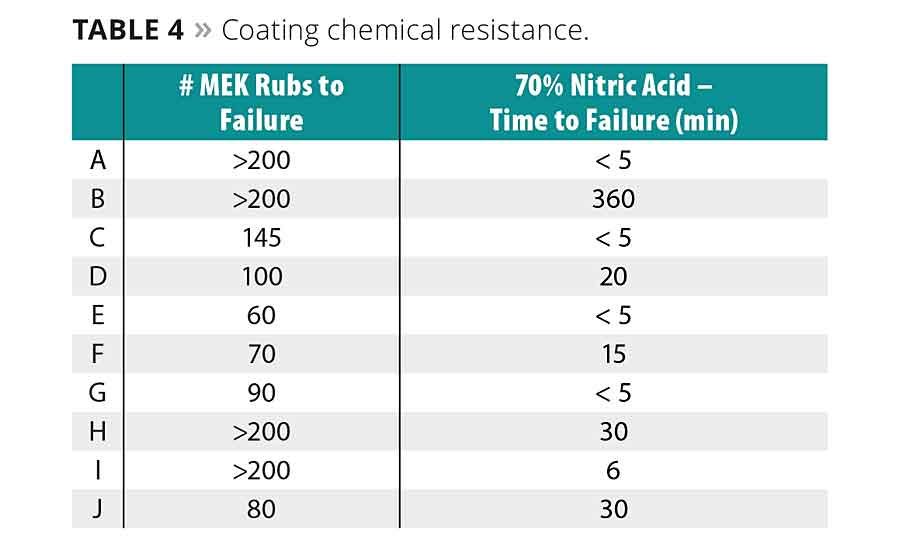
Table 4 reports the coatings’ resistance to solvents and chemicals using methyl ethyl ketone and 70% nitric acid as test substances. Most oligomers had good to excellent resistance to MEK solvent. The added hydroxyl or carboxylic acid groups in H-I seem to improve this result. Higher molecular weights may also have an inverse relationship on solvent resistance, as seen by comparing D-F or considering J. Such higher-molecular-weight oligomers are more likely to have more or larger pockets within the cured, cross-linked network where solvents may penetrate.
The 70% nitric acid resistance data are much more varied. The urethane containing B performed substantially better than the other oligomers. D, F, H and J also showed improved resistance when compared with the A and C standard oligomers.
Surface Tension and Adhesion
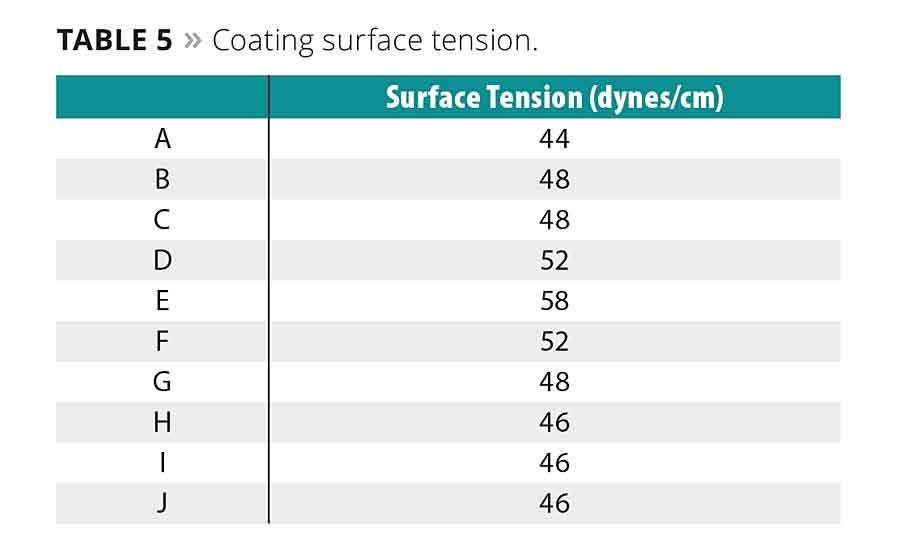
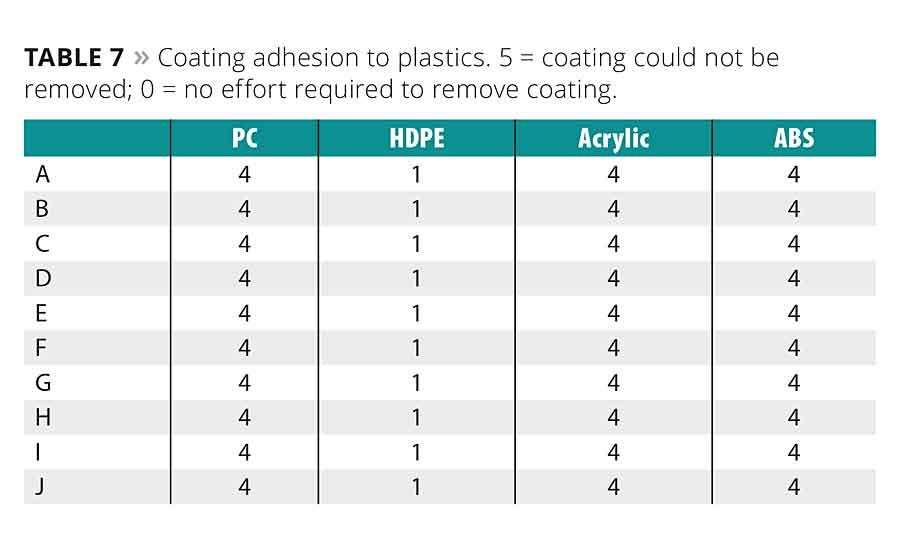
The surface tension of the oligomer coatings is given in Table 5. Surface tension can play a role in a coating’s wetting properties, as well as ultimate adhesion to a substrate. Adhesion data for the coatings to glass, metals and plastics are shown in Tables 6 and 7.
There is little difference in adhesion to plastics between oligomers. With the exception of the HDPE, all adhere quite well to the substrates. The adhesion to glass and metal is similarly good across all oligomers, with the standard A-C oligomers having slightly poorer adhesion overall.
Pigmented Ink Properties
Preparation
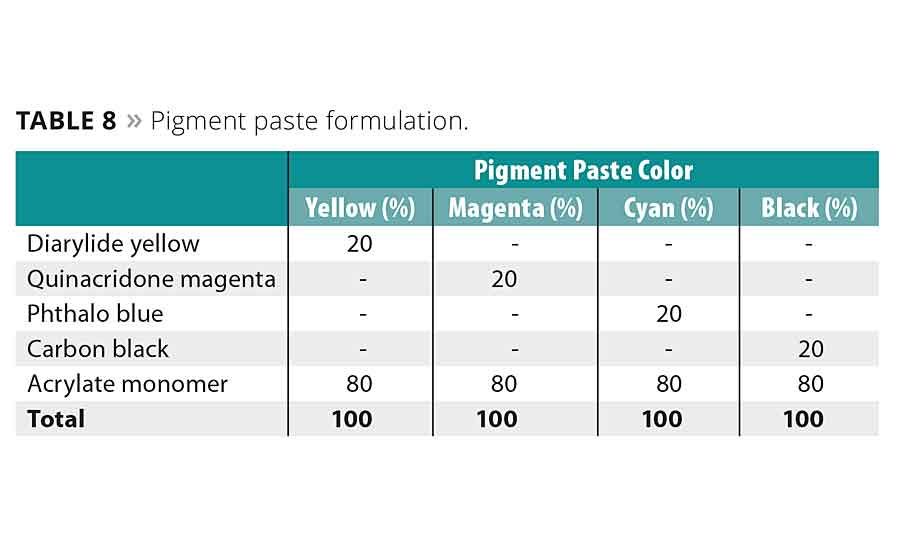
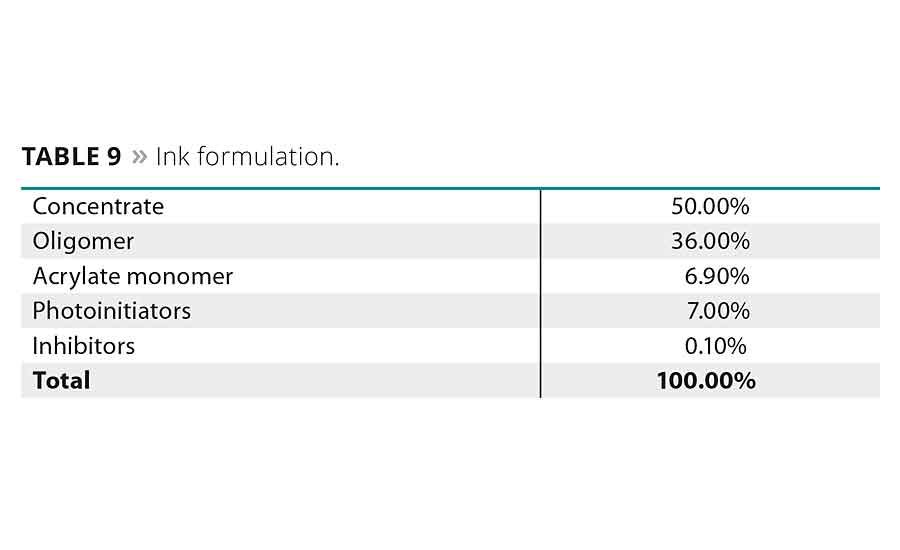
The pigmented inks were prepared in a two-step process. First, a pigment paste was created by mixing the respective pigment into acrylate monomer under high shear until an appropriate level of dispersion was reached. The second step involved adding the oligomer, more acrylate monomer, photoinitiators and polymerization inhibitors to this pigment paste and mixing until homogeneous. No pigment stabilization additives or surfactants were added to ensure any stability results would be solely due to the oligomer used.
The formulas of the pigment pastes and inks are given in Tables 8 and 9, respectively. The formulas of both were adjusted so that the final pigment load in the ink would be 10%.
Ink Viscosity and Stability
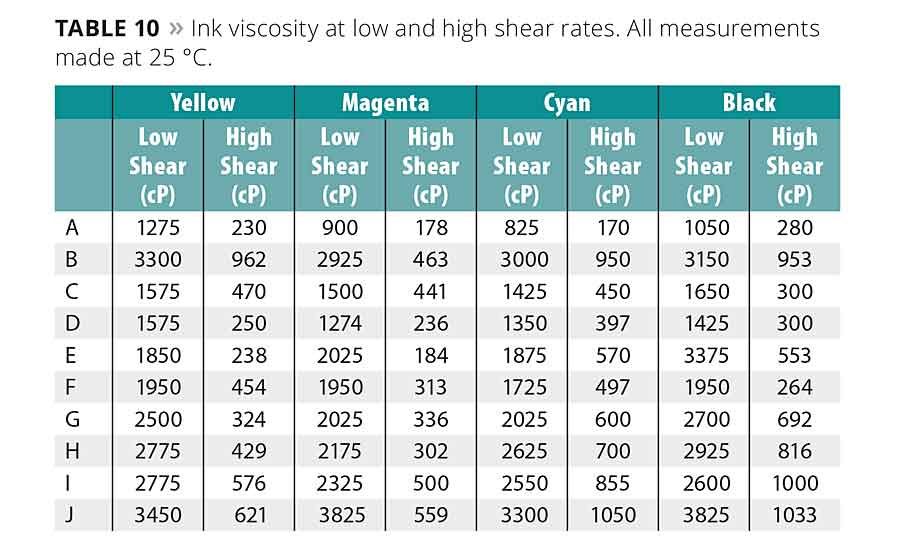
The final ink viscosities as measured under low shear (50 s-1) and high shear (2600 s-1) can be found in Table 10. The viscosities are fairly consistent across colors and correlate well to the starting oligomer viscosity. In many cases, the high-shear viscosity is low enough to be suitable for various printing and ink applications.
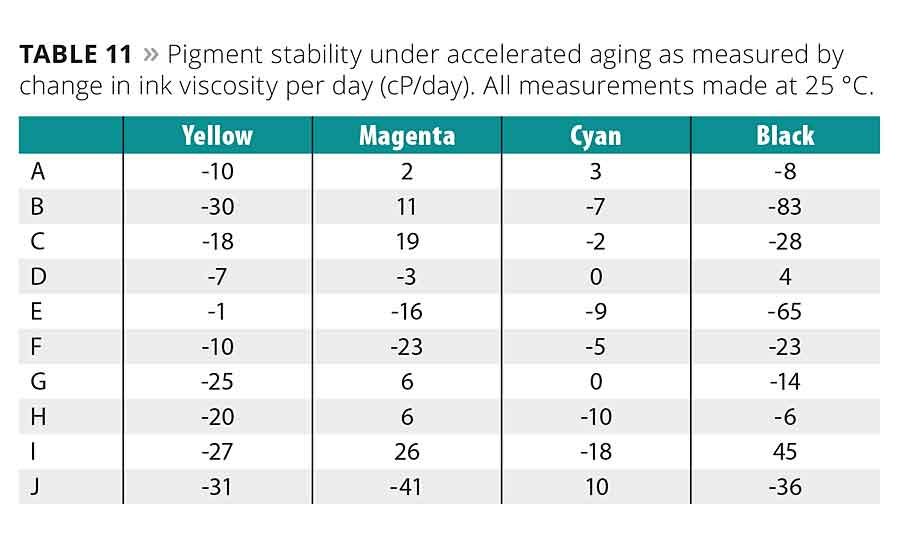
To compare the relative stability of the pigmented inks, small samples were placed in a 60 °C oven for accelerated aging testing. The viscosities of the samples were measured again periodically for a time period greater than 20 days. The data in Table 11 is the rate of change in the low-shear viscosity over this time period in cP/day.
On the whole, the inks’ stability looks to have a mostly inverse relationship with the oligomers’ viscosity and molecular weight, implicating wettability and steric hindrance properties. The low-viscosity, low-molecular-weight oligomer D shows the best overall stability being the most or second most stable in all colors. A and G were also fairly stable. The oligomers having the worst overall stability were the high-viscosity, high-molecular-weight J as well as I. This implies that the addition of the carboxylic acid group in I had a destabilizing effect on pigment stability. The stability of the high-viscosity urethane acrylate B was also poor.
In regard to each ink color individually, the stability of the yellow inks was more closely associated with oligomer viscosity than molecular weight. The addition of other oligomer functional groups, i.e., the hydroxyl groups, decreased the inks’ stability. The magenta inks were the opposite, being mostly associated with molecular weight. The added hydroxyl groups had an overall stabilizing effect in these inks.
All the cyan inks showed good stability, with the low-viscosity, low-molecular-weight oligomer inks being slightly better. The D- and G-based cyan inks showed no change in viscosity over time. Stable black inks were hard to achieve except with A, D, G and H, which confirms the general trend seen with the other color. The presence of hydroxyl groups had little to no effect on the stability of the cyan and black inks.
Conclusion
In this article, we have described several new dendritic acrylate oligomers with varying structure that may find uses in coatings and pigmented inks. These oligomers have been shown to have good cure speed, resistance to oxygen inhibition and physical properties that are equal to, if not better, than other oligomer types typically used for these applications. We further investigated how oligomer structure may impact the stability of ink formulations based on these oligomers. Although the specific oligomer characteristic that resulted in the best stability somewhat varied by color, it was found that lower-viscosity and lower-molecular-weight oligomers generally resulted in more stable inks.
References
1 Vernerdakis, T.G. Coatings Technology Handbook, Third Edition, 2005, 76-1-76-19.
2 Lu, W.H.; Xu, W.J.; Wu, Y.M.; Zhou, X.; Lu, Y.B.; Xiong, Y.Q. Prog. Org. Coat., 2005, 56, 252.
3 Xu, D.-M.; Zhang, K.-D.;Zhu, X.-L. J. App. Polmer Sci., 2004, 92, 1018.
4 Dymax Press Release, “High-Functionality Oligomer Exhibits Superior Chemical and Temperature Resistance”, March 18, 2013.










Report Abusive Comment