Today, electron beam (EB) systems have become a widely used method of manufacture. They are recognized for their ease of handling, operation and focus on safety. Electron beam processing can be done under vacuum, partial vacuum or nonvacuum conditions. High-vacuum conditions result in fewer gaseous molecules between the electron lamp and the targeted substrate, which results in less scattering and a tighter beam.
Low-energy EB technology is defined as beam operations with an accelerating voltage of less than 300 kV. Low-energy EB is used in many surface curing, crosslinking, scission, grafting and surface sterilization applications. Low-energy EB systems also use tungsten filaments to generate an ongoing stream of electrons under vacuum that are propelled through a titanium foil window onto a targeted substrate. Standard, commercial low-energy EB systems range from 30" (762 mm) to 108" (2743 mm) in width, depending on the design width of the electron beam. In the field of narrow web processing, the sealed low-energy ebeam lamps range in window width from 220 mm to 400 mm. Vacuum pumps are not needed for compact, sealed ebeam systems, which do not require filament or foil replacement. Like a light bulb, ebeam lamps are replaced in full as a spare part. This is a crucial benefit in applications where service time is vital.
EB Curing, Crosslinking, Surface Sterilization and Grafting
In very simple terms, EB enables cut, link and paste:
- Cut: Sterilization and controlled molecular weight reduction of polymers;
- Link: Crosslinking of polymers or curing of liquid inks to solid coatings;
- Paste: Grafting of a second polymer species to a trunk polymer.
Compared with conventional hot air drying methods, EB curing requires a much smaller footprint and less operating labor, and drastically reduces full cure time for coatings, inks and adhesives to less than a second. EB curing systems provide environmental benefits because they eliminate most volatile organic compounds, use little energy and operate at near room temperature. Contrary to free-radical UV curing, which requires a photoinitiator (or at least some photo-reactive moiety incorporated into the oligomer), free-radical EB curing occurs without adding photoinitiator. The accelerated electrons produced by EB systems have sufficient energy to open reactive chemical bonds within the materials themselves without need for photoinitiators. The bond opening (cut) leads to the generation of free radicals that initiate polymerization. The ability of EB to create radicals directly in the material also makes them useful in a variety of other processes.
In 1952, Arthur Charlesby discovered that the radiation crosslinking of polymers such as polyethylene could be accomplished with EB. Today, a very well-established application in this area is the crosslinking of polyethylene-based films to provide heat-shrink properties for meat and cheese packaging, pioneered by Cryovac in the late 1950s. Around the same time, Raychem started introducing cables and wires for telephone switchboards with EB crosslinking, eventually growing into a billion dollar business. Other well-established applications for EB crosslinking include high-performance pressure-sensitive adhesives and pre-vulcanization treatment of the inner rubber cord layer of automotive tires.
Scission occurs when polymer chains are broken and fail to recombine. The net result of polymer scission is a reduction in molecular weight. Industrial processes for EB scission (with the exception of sterilization) are less common than curing or crosslinking. Examples of EB scission are the processing of polytetrafluoroethylene (PTFE) to make low-molecular-weight fragments for use in waxes and lubricants, and the irradiation of pulp where the modification of cellulose is needed.
Electron beam-induced graft copolymerization (EIGC) occurs when radicals formed in and on a polymer substrate become a site for initiation of monomer polymerization or a site of attachment for a fully functional group – generally also referred to as the “grafting-from” and “grafting-to” approach. The net result is that two dissimilar polymers are covalently joined to form a new copolymer material. EB grafting is less well known than curing or crosslinking but is an important process for creating new functional materials. It can be used to modify the properties of polymer films, gels, fibers, beads or membranes. Applications for EB grafting include the production of polymer membranes tailored for specific separation or purification processes, thermo-responsive surfaces and vitamin E-stabilized implants.
Sterilization with high-energy EB systems is well established. Major alternatives to high-energy EB sterilization technology include gamma irradiation, peroxide and ethylene oxide, which can be expensive to operate and hazardous to humans and the environment. Using low-energy “ebeam lamps” for the surface sterilization of aseptic packaging and medical devices is quickly emerging as a preferred method for surface sterilization as it eliminates chemical and water usage, enabling a reduction of the energy and waste treatment expenses typically associated with chemical sterilization processes. In 2015, Tetra Pak, a multinational food packaging and processing company, announced the global launch of an ebeam-equipped E3 platform of filling machines that contain compact low-energy electron beam lamps. “The lamps replace hydrogen peroxide as the method to sterilize the packaging material. The use of electron beam allows Tetra Pak customers to reduce their environmental impact while at the same time increase production speed (by over +60%) and reduce operating costs.”1
New Opportunities for Low-Energy EB Technology
Challenges inherent to using full-production-scale low-energy electron beam systems for R&D and proof-of-concept purposes are numerous. Full-scale EB systems were never intended to run research – volumes and consumables quickly add up – and obtaining access to a commercial EB outside of prescheduled plant shutdown can be a challenge and disruptive to the overall new product development process. Performing those tasks at a service center are no less tedious: travel, limited flexibility onsite as well as not having the tools and infrastructure for the particular research with immediate feedback make it a high entry-barrier approach only palatable for the truly committed or zen-like personalities.
In 2001, Advanced Electron Beams (AEB) introduced a compact lab system (Applications Development Unit, or ADU for short) that incorporated a 10-inch wide, hermetically sealed tube lamp that required no external vacuum pumps, or on-site foil or filament replacement. Over 70 of these ADUs were installed globally, many of which are still in operation today. This compact system was the first of its kind. It had a maximum operating voltage of 150 kV. Although it had limited penetration capabilities, it provided laboratories and formulators with an excellent tool to support EB products, processes and applications development. AEB is no longer in business.
In 2012, a new EB laboratory system made by COMET ebeam Technologies, a business of the COMET Group, was commercialized. COMET AG is a global leader in the manufacture of industrial X-ray sources and systems for safety and non-destructive testing. The EBLab, which uses COMET’s sealed lamp technology, is now distributed globally and contains a 200 kV lamp. A 300 kV version of the ebeam lamp is also being developed and is expected to be released in 2016. The EBLab200 uses patented, sealed lamp technology, which eliminates the need for external vacuum pumps and foil changes that are part of the operation and maintenance scheme for larger-scale, non-integrated lamp EB systems.
The sealed lamp (Figure 1) is integrated with a high-voltage cable and power supply to form an ebeam engine. This lamp technology allows processing voltages ranging from 80 to 200 kV, which is a significantly wider range of power than previously available in other lab-scale systems. The ebeam lamp is also being integrated by COMET’s PCT Engineering Systems LLC division into shielded inline reel-to-reel and 3D curing system configurations for product development and production purposes. Therefore, the transfer of product development from EBLab to full-scale production systems is straightforward and immediate.
To promote low-energy ebeam technology awareness along with access and usage of the EBLab-200 system, in early 2015 COMET set-up EBLab “ateliers” workshop areas at its global headquarters in Flamatt, Switzerland, and regional facilities in the United States, China and South Korea. In June 2015, COMET entered into partnerships with Georgia Power in Atlanta and Wells Fargo Bank to promote low-energy ebeam technology. Georgia Power is the largest of the four electric utilities owned and operated by The Southern Company. A fully operational EBLab Unit was installed at Georgia Power’s Customer Resource Center (CRC) and will be used for a number of co-sponsored workshop events in the second half of 2015. All EBLab Workshop participants interested in acquiring a COMET EBLab or PCT integrated system now have leasing options with Wells Fargo in the United States.
In addition to EBLab Workshops scheduled to take place at Georgia Power’s CRC in 2015, COMET and PCT are actively promoting the EBLab and electron beam technology to universities and institutional research facilities throughout the Americas via programs designed to build inter-institutional collaborations based on core areas of electron beam application expertise. The hope for North America is that inter-university collaborations thriving around emerging ebeam applications be established with EBLab institutional partners encouraged to build curriculums focusing on low-energy electron beam technology. Collaborations thus far have generated a number of research projects for ebeam applications resulting in peer-reviewed papers and articles.
Conclusion
Low-energy EB processing is a technology in evolution. After decades of refinement and integration it continues to offer the potential of significantly enhancing production efficiencies and environmental benefits for manufacturing and brand owners focusing on sustainability, consumer safety, cost competitiveness and product differentiation. Optimizations focusing on smaller footprint, availability of access and affordability will continue to popularize interest in low-energy electron beam for emerging product applications. Partnerships with key stakeholders through the low-energy electron beam value chain, from public utilities to universities, will continue to be indispensable before ebeam becomes a common household word.
Acknowledgements
The author would like to acknowledge Michael Bielmann, Ph.D. and Kaspar Zimmerli of COMET Group, along with John Salkeld and Steve Lapin, Ph.D. from PCT Engineered Systems LLC for their contributions to this article. Acknowledgement is equally made to Georgia Power’s CRC as a partner for the promotion of low-energy electron beam technology.
References
1 Eagle, J. Tetra Pak launches eBeam to replace hydrogen peroxide sterilization of its packaging material. https://plus.google.com/110511156977251851193/posts, 23-Jun-2015.
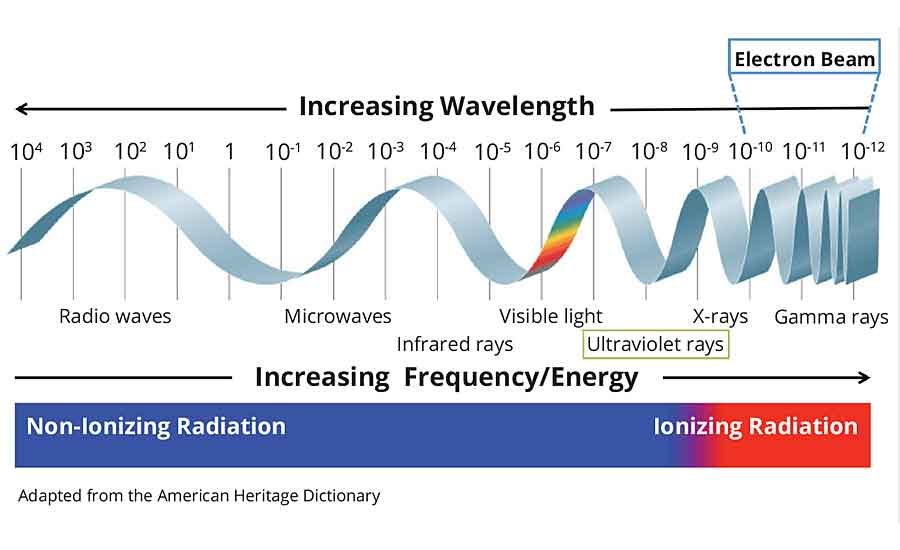
Electron Beam vs. Ultraviolet Curing Technology
Electrons are stable, subatomic particles with a charge of negative electricity that are found in all atoms and act as the primary carrier of electricity in solids. Electrons equally have the ability to pass through many materials opaque to light. Electron beam and x-rays have more energy than ultraviolet (UV) light waves and are considered ionizing radiation.
Contrary to free-radical UV curing, which requires a photoinitiator (or at least some photo-reactive moiety incorporated into the oligomer), free-radical EB curing occurs without adding photoinitiator. The accelerated electrons produced by EB systems have sufficient energy to open reactive chemical bonds within the materials themselves without the need for photoinitiators. The bond openings lead to the generation of free radicals that initiate polymerization. The ability of EB to create radicals directly in the material also makes it useful in a variety of other processes.






Report Abusive Comment