With rapid industry development, environmental protection issues are being taken more and more seriously all around the world, especially in China. In early 2015, the Chinese government claimed that solventborne coatings manufacturers began to pay a VOC tax as high as 4%. Because powder coatings are almost free of VOCs, this will provide powder coatings manufacturers more development opportunities and support from local government.
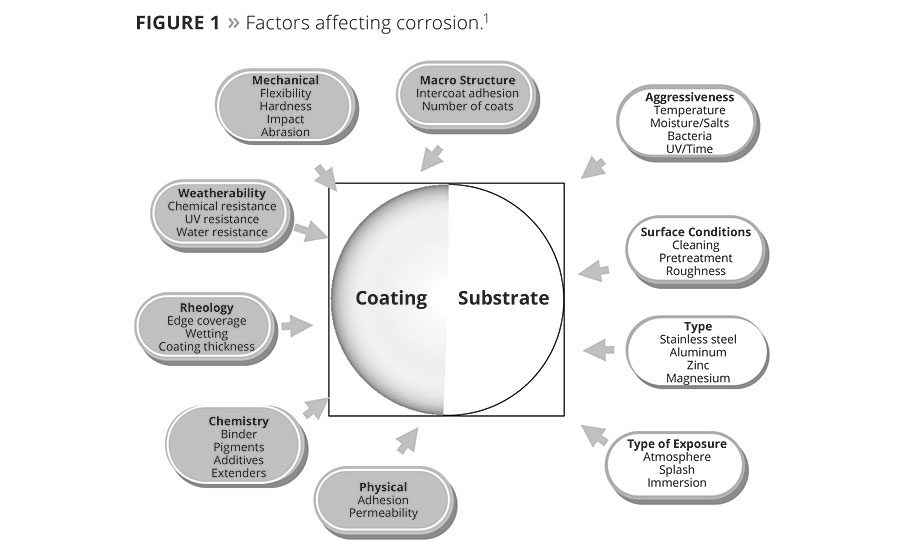
Powder coatings are widely used in various fields, such as architecture, automobiles, pipelines, IT mobile and telecoms, and domestic appliances. For powder coatings, the fight against corrosion has not been stopped. Although the oil and pipeline industry has developed a reliable cathodic protection method, they also experience coating failures. The main reason for this lies in a number of factors.1 The coating system includes binders, pigments and extenders, additives, the type of substrate, pretreatment, curing, coating thickness, adhesion, and the external environment, such as the temperature, moisture, bacteria and UV. All the corrosive relationships are displayed in Figure 1.
What powder coating manufacturers can contribute to corrosion resistance mainly lies in the coating’s chemistry, which means the reasonable selection of binders, pigments and extenders, and anticorrosion additives. These influencing factors will be discussed in this article.
Binders
Polyester, acrylic, polyurethane and epoxy resins are mainly used in powder coatings. Polyester is prone to hydrolysis in moist environments due to the ester group. Acrylics have seen failures in acid rain due to hydrolysis as well. Polyurethanes can be applied as anticorrosion coatings, but the price is high. Because of their moderate price and excellent anticorrosion properties, epoxy resins are a better choice for anticorrosion coatings. Figure 2 shows bisphenol-A epoxy as an example.
The following factors contribute to bisphe-nol-A epoxy’s anticorrosion properties:
- The pendant hydroxyl group offers good adhesion to the metal and cohesive force;
- The ether bond in the backbone offers chemical and alkali resistance;
- The inexistence of an ester bond in the epoxy structure gives better water resistance;
- High crosslink density of the cured product gives a better barrier to the substrate.
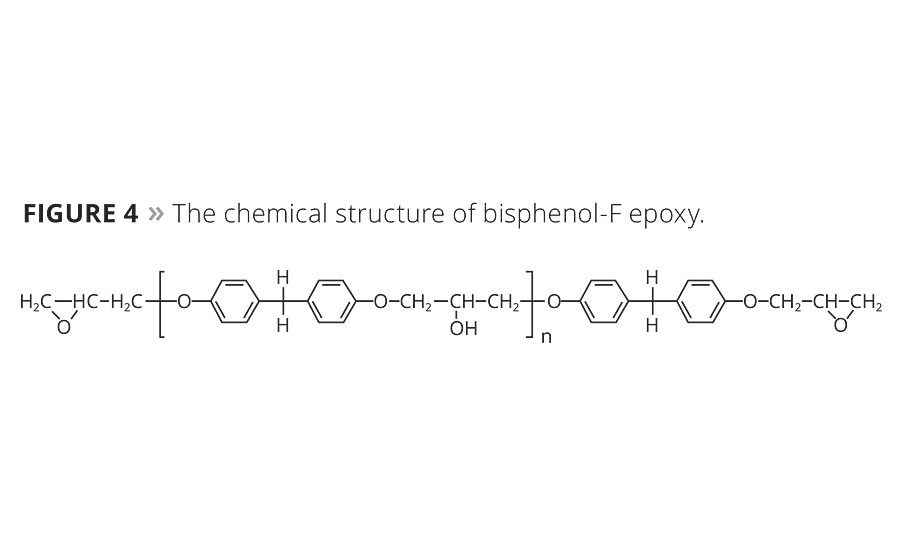
Besides bisphenol-A epoxy, novolac-modified epoxy and bisphenol-F epoxy can be used in anticorrosive powder coatings as well. The corresponding chemical structures are shown in Figures 3 and 4.
It can be seen from the chemical structure that the enhanced corrosion resistance of novolac-modified epoxy originates from a high number of epoxy groups and aromatic rings per molecule, as compared with bisphenol-A or bisphenol-F epoxy. This results in the tightly crosslinked structure in novolac-modified epoxy resins, but they are seldom used individually due to the brittleness of the cured product. Novolac-modified epoxy is generally combined with bisphenol-A epoxy to achieve excellent mechanical and anticorrosive properties in heavy anticorrosion powder coatings. Bisphenol-F epoxy for powder coatings is mainly for the improvement of the flowability, and can be combined with bisphenol-A epoxy as well.
Molecular Weight
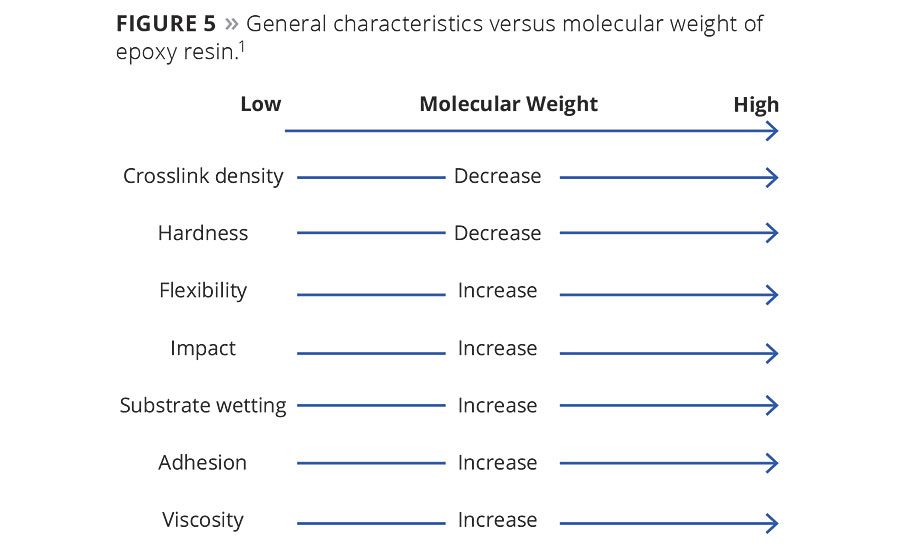
In addition to the chemical structure, molecular weight is another important factor that affects various properties of epoxies. Figure 5 shows the dependence of various properties of epoxy on molecular weight. Compared with high-molecular-weight epoxy, coatings made from low-molecular-weight epoxy can have greater crosslink density due to more epoxy functional groups, which results in coatings with higher hardness, decreased flexibility and lower impact resistance. Unreacted low-molecular-weight epoxy has lower hydroxyl groups than unreacted high-molecular-weight epoxy, which can also cause reduced substrate wetting and adhesion on metals. Considering the comprehensive performance of powder coatings, epoxy molecular weight must be within a certain range. The epoxy equivalent weight (EEW) of epoxy should be 700-900 g/eq and have narrower molecular weight distribution for heavy anticorrosive coatings.
Binder Curing Agents
Binders consist of the resin and curing agent for thermosetting powder coating formulations. The epoxy curing agents commonly used in powder coatings include imidazole, amine (dicyandiamide and aromatic amine), anhydride and phenolic.
Internal stress and poor mechanical properties exist in powder coatings cured with imidazole due to its low activation energy and violent curing reaction. Imidazole is rarely used individually as a curing agent, but rather as a catalyst in epoxy powder coatings. Dicyandiamide is one common curing agent in powder coatings due to its integrated properties and cost performance. Several aromatic amines can also be applied in powder coatings; for instance, Z. Wang et al.2 compared the corrosion resistance of coatings cured with three different kinds of aromatic amines -diaminodiphenylsulfone, metaphenylene diamine and phenolic aldehyde amine. It was found that the coating cured with diaminodiphenylsulfone showed the best anticorrosion properties in a hot, 10% sulfuric acid solution. The application of anhydride is limited to some extent because of storage stability and irritating volatiles. Phenolic curing agents have become the first choice in heavy corrosion environments, such as oil and pipeline fields, owing to their low bake, fast reaction and excellent anticorrosion properties.
Pigments and Extenders
Large amounts of anticorrosive pigments or extenders can suppress corrosion by barrier protection, sacrificial properties or inhibitive reaction according to the protection mechanism.
Barrier protection is offered by lamellar extenders through thickening the coating layer and impeding the permeability of corrosive substances to the surface of the metal substrate. Lamellar extenders, widely used for anticorrosive powder coatings, are micaceous iron oxide, sericite and glass flake.1 Corrosive substances tend to migrate straight through the coating with spherical pigments, whereas coatings formulated with lamellar extenders impede the transport of corrosive substances by providing a tortuous path of diffusion. In powder coatings, the extenders are melt blended through extrusion, which destroys the original shape of these lamellar extenders, and thus limits their usage in powder coatings.
In principle, all metals that are electrochemically more active than the substrate to be protected can be applied as sacrificial pigments in anticorrosive coatings. At present, metallic zinc particles are the most widely used among sacrificial pigments. The best anticorrosive performance of spherical zinc particles is reported to be obtained with an average diameter of 2 µm.3 The protective mechanism of metallic zinc involves insoluble corrosion precipitations such as ZnFe2O4 and basic zinc carbonate products. In particular, the anticorrosive performance of zinc particles is significantly enhanced when traditional spherical zinc is replaced by flaked zinc, which possesses shielding properties and parallel lapping performance in powder coatings.
Inhibitive pigments in powder coatings may be classified according to their effect on the anodic and cathodic reactions. Cathodic inhibitors, such as inorganic salts of magnesium and aluminum, suppress corrosion at the cathode by forming insoluble deposits with hydroxyl ions in neutral environments. Anodic inhibitors, such as inorganic salts of phosphate or silicate, and hydroxide compounds, form a protective oxide film on the metal surface. In the case of insufficient addition of anodic inhibitors, an undesirable anode to cathode area will be formed, which increases the rate of corrosion.4 At present, phosphates containing pigments such as zinc phosphate and magnesium phosphate are the most widely used inhibitive pigments. In addition, another group of inhibitive pigments are spinel-type pigments based on mixed metal oxides, which have reduced toxicity compared with most pigments.
Additives
In principle, all additives that contribute to the adhesion of the coating to metal can improve the anticorrosion properties. Some silanes or modified silanes have a good effect on the interface between the coating and metal. There are other types of adhesion promoters other than silanes available for powder coatings that have a high concentration of amino, carboxyl or hydroxyl functional groups, which can be incorporated into the coating before extrusion. Also, some studies5,6 have been done on polyaniline mixed with epoxy to enhance anticorrosion properties.
Perspective
With more focus on environmental protection all over the world, powder coatings will have greater application prospects in the near future. But so far, powder coatings can seldom be used in C5 and C6 corrosion categories due to various limitations. There is still much work for powder coating technologists, application equipment suppliers and related pretreatment specialists. Among anticorrosive additives, “green” corrosion inhibitors with decreased toxicity are a growing trend. It has been discussed that some of these corrosion inhibitors7,8 displayed excellent inhibitive effects in liquid paint under a variety of corrosive environments. Even though non-toxicity and biodegradability are the major advantages for these inhibitors, various technologic boundaries exist. Further research and effort are still needed to employ the green inhibitors widely at an industrial level, especially in powder coatings.
References
1 Sorensen, P.A.; Kiil, S.; Dan-Johansen, K.; Weinell, C.E. Anticorrosion Coatings: a Review. Journal of Coatings Technology and Research2009, 6(2) 135-176.
2 Wang, Z.; Han, E.; Liu, F.; Ke, W. Effect of Different Curing Agents on Cure Reaction and Exposure Resistance of Phenolic-Epoxy Resins in Hot Acid Solutions. Corrosion2010, 66(7) 1-9.
3 Kalendova, A. Effects of Particle Sizes and Shapes of Zinc Metal on the Properties of Anticorrosive Coatings. Progress in Organic Coatings2003, 46(4) 324-332.
4 Arya, C.; Vassie, P.R.W. ‘‘Influence of the Cathode-to-Anode Ratio and Separation Distance on Galvanic Corrosion Currents of Steel in Concrete Containing Chlorides. Cement and Concrete Research, 25(5), 1995, pages 989-998.
5 Kalendova, A.; Vesely, D.; Stejstal, J. “Organic Coatings Containing Polyaniline and Inorganic Pigments as Corrosion Inhibitors. Progress in Organic Coatings2008, 62(1) 105-116.
6 Radhakrishnan, S.; Sonawane, S.; Siju, C.R. Epoxy Powder Coatings Containing Polyaniline for Enhanced Corrosion Protection. Progress in Organic Coatings2009, 64(4) 383-386.
7 Kesavan, D.; Gopiraman, M.; Sulochana, N. Green Inhibitors for Corrosion of Metals: A review. Chemical Science Review and Letters, 1(1), 2012, pages 1-8.
8 Arthur, D.E.; Achika, J.; Ameh, P.O.; Anya, C. A Review on the Assessment of Polymeric Materials Used as Corrosion Inhibitor of Metals and Alloys. International Journal of Industrial Chemistry2013, 4(2).
For more information, e-mail wenjuan.gong@akzonobel.com.








Report Abusive Comment