Four manufacturing processes have gained industrial significance worldwide for the production of iron oxide red pigments. The two most important processes for the production of red iron oxide pigments, in terms of volumes, are the Laux process and the Penniman process, which together account for more than 90% of the worldwide demand for synthetic red iron oxides. However, the pigment properties and environmental impact of the two processes differ significantly.
Pigments from the Laux process offer a wide color range with particular strengths in medium and bluish red color shades. The relatively hard consistency of the primary Laux particles has its most positive effect in grades with large particle diameters. Furthermore, the pigment formation via a calcination step leads to good milling stability, particularly in bright reds with particle sizes lower than 0.4 µm.
Coloristic properties are not only determined by particle size but also by the morphology of a pigment, which differs due to different manufacturing processes. Thus it is possible to manufacture very bright red pigments with yellow undertone, exemplified by the Penniman process.
The traditional manufacturing process for iron oxide red using the Penniman route is considered particularly challenging due to the formation of gaseous nitrogen oxides and water-soluble nitrate and ammonium compounds in the wastewater (Figure 1).
LANXESS has proven that the conventional Penniman reaction process gives rise to the formation of significant amounts of laughing gas (nitrous oxide, N2O). Nitrous oxide accounts for 5-6% of the total global emissions of greenhouse gases and is produced by 500-600 companies around the world. It occurs mainly as a byproduct of the manufacture of nitric acid and has an almost 300 times higher climate impact than CO2. As part of the World Climate Summit held in Paris at the end of 2015, the German Federal Environment Ministry proposed an initiative that by 2020 global nitrous oxide emissions should be eliminated by the use of efficient catalyst technology.
LANXESS took on the challenge and developed and implemented the Ningbo process in its new production plant in China to manufacture bright iron oxide reds in a sustainable way (Figure 2). This production process recycles emitted gases, including nitrous oxide, by using a complete catalytic decomposition of N2O to form naturally occurring nitrogen, oxygen and water. This results in a reduction of over 70% CO2 equivalents, relative to conventional Penniman red technology. Furthermore, using a multistage wastewater treatment process, more than 80% of the wastewater is cleaned and recycled back into the process. The remaining 20% of the wastewater is virtually free of nitrates and only contains dissolved sulphate, which can be re-used in the process.
It is worth stressing that conventional Penniman producers still continue to emit the greenhouse gas N2O unrecycled directly into the environment.
New Shades of Red
In the most modern iron oxide production plant located in Ningbo, China, approximately 25,000 tons of bright, yellow-shade red pigments will be synthesized and are likely to be of particular interest for paint and coating applications. These “New Red” pigments will be marketed in the future under the Bayferrox brand. Table 1 summarizes some technical characteristics of the New Red series.
In addition to the synthesis capacity of the Ningbo facility, the plant will also have a state-of-the-art mixing and milling plant with a capacity of around 70,000 tons. By blending the synthesized red pigments it is possible to reach both new and established color shades (Figure 3).
The Ningbo process is made in several steps. Primary raw materials are iron (in the form of steel), nitric acid, water (in the form of steam), ferrous sulphate and air. To achieve defined and clean color shades it is necessary to grow the pigment particles around a defined seed. Increasing the particle size around the seed leads to an optimum color saturation point, after which the pigment begins to lose chromaticity (Figure 4).
Numerous factors have a direct influence on the pigment growth. In the case of bright yellow-shade red iron oxide pigments, it is possible to widen the color development curve to reach higher a* and b* color values. Furthermore, through specific measures, the build up curve can be precisely stopped at the desired color shade to enable a number of targeted color spaces to be achieved. The influencing factors are complex and need to be applied in the right way and at the right dosage. Raw material selection and the seed quality are of fundamental importance. In addition, the control of the reaction progress and the reaction conditions must also be coordinated. No single factor is decisive; a combination of different factors will result in the highest pigment quality.
The iron oxide seed preparation, in particular, represents a special challenge. Only a defined particle shape and narrow particle size distribution ensure a uniform pigment growth (Figure 5). For this reason, the raw material quality is of fundamental importance. Iron scrap can contain a lot of accompanying elements. Used as a raw material for the production of iron oxides, some of these elements can have a negative influence on the coloristic properties of the pigment. Using the Ningbo process optimization, it is possible to significantly lower the amount of disruptive side elements within the crystal lattice of the final pigment.
In addition to efficient reduction of waste materials, changes in the process management lead to pigments covering new color spaces that surpass all previously available iron oxide red pigments in the market. This extraordinary color development can be predicted by just looking at the pure pigment powder.
The absorption of inorganic color pigments is mainly determined by their elemental composition and crystalline structure. The size of the primary pigment particles is, however, of critical importance for light diffusion.
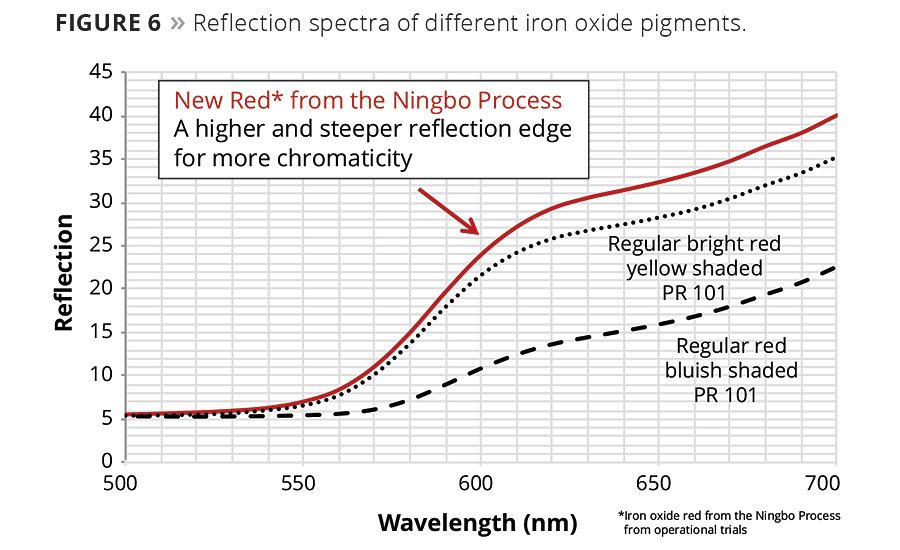
Iron oxide pigments have a relatively unsaturated color shade compared to some color pigments produced using other chemical processes. This can be explained by looking more closely at the physical boundary conditions imposed by nature. Due to the short distance between the iron ions of only 2.89 nm within the crystal lattice, the excitation in visible light triggers a d-d electron transition. In the reflection spectrum a rather flat edge with a clear and distinct shoulder is visible. The higher and steeper the reflection edge the more saturated the shade. A comparison of the spectra of a regular blue-shade red and a regular bright yellow-shade red compared to one of the new pigments from the Ningbo process is illustrated in Figure 6. The reflection curve of the pigment from the Ningbo process is clearly steeper, and this is directly related to higher chromaticity.
For coloristic evaluation in final coatings systems, bright, yellow-shade iron oxide reds from various manufacturing processes were selected and compared against each other. The chromaticity values C* were determined in a long-oil alkyd system, excluding all other paint ingredients such as wetting and dispersing additives. Only then can the color development of the pure pigment be displayed.
In both full shade and reduction (1:5 with TiO2), the newly developed iron oxide reds from the Ningbo process show the highest chromaticity value C*(Figure 7). It is noticeable that pigments from the different manufacturing processes do not show consistent C* values in full shade and reduction. Higher C* values in full shade often give low values in reduction and vice versa. The results illustrated for the Ningbo process were made using material from an operational trial reactor in Krefeld, Germany, which was operated according to the Ningbo process.
Efficient downstream grinding technology, which increases the share of primary particles in the pigment, improves the incorporation ability into liquid systems significantly. This process is called micronization and was first introduced by Bayer in Germany in 1964, setting a new milestone for the coatings industry.
The iron oxide red pigments from the Ningbo process are softer than Laux calcined pigments and therefore require an adapted form of micronization. In the milling plant in Ningbo, China, state-of-the-art grinding technology is used, whereby the particles are ground continuously to a reproducible size.
It is desirable to keep the energy input in the dispersion as low as possible. In the case of the New Red series from the Ningbo process, optimum results can be achieved by using high-speed dissolvers. The quality of the dispersion is excellent and comparable with established red iron oxides from the LANXESS Bayferrox 100 M series.
This can be clearly illustrated by photographic means. The white dots on the grinding gauge images represent protruding particles after 15 min dispersing with a high-speed dissolver in a long-oil alkyd system. The scaling ranges from 0 to 100 µm.
Figure 8 illustrates the grind values of an iron oxide from the Ningbo process compared to a regular calcined iron oxide red.
Narrow Particle Size Distribution
The different color spaces of iron oxide reds are due, among other things, to differences in the particle morphology and the particle size distribution. The exact color parameters cannot, however, be predicted using a direct correlation with the particle morphology or the particle size distribution. Light, yellow-shade red pigments tend to exhibit a smaller particle size and an especially narrow particle size distribution, while darker red pigments are significantly larger. Due to light scattering, the size of the primary particles exerts an influence on the chromaticity, tinting strength and opacity.
The interaction between visible light in the wavelength range of 380 to 780 nm and pigment particles reaches an optimum if the particles are in the magnitude of half the wavelength of the absorbed light. In the case of red iron oxide pigments, this optimum is at a particle size of 250 to 300 nm. Smaller particles support the red value a*. Iron oxides from the Ningbo process contain a high amount of fine particles in the optimum particle size range. The determination of particle size distribution, by means of the laser diffraction method, is an indirect method that calculates the values from a formula that contains, among other things, the complex refractive index for hematite. The refractive index is not yet sufficiently defined and distinctive. Therefore, the values should only be interpreted comparatively. The measurement is related to the particle volume, i.e. larger particles are represented disproportionately.
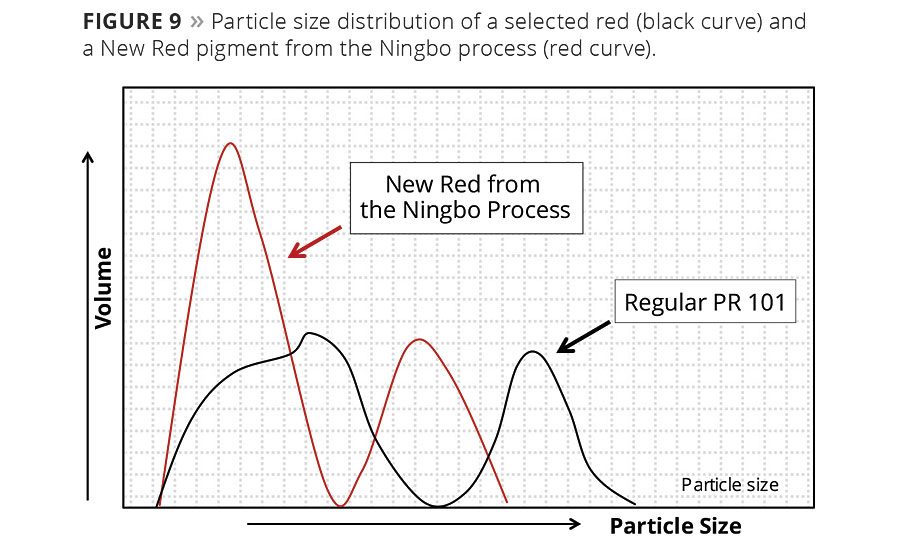
Figure 9 shows the volume-related particle size distribution of a regular calcined hematite pigment PR 101 (black curve) compared to an iron oxide from the Ningbo process (red curve). The optimum area of particles is larger, which indicates additional chromaticity.
Results at a Glance
In addition to highly sustainable production, the Ningbo process provides iron oxide red pigments with exceptional coloristic behavior that exceeds any currently available iron oxide pigment in the market. This is due to process optimization, which enables a defined particle shape to be generated coupled with narrow particle size distribution and uniform pigment growth.
The newly developed Bayferrox New Red pigments are characterized by excellent incorporation ability, even when using low-shear dispersing forces. The dispersibility determined on a grinding gauge is similar to the benchmark Bayferrox 100 ‘M’ micronized series.
Combinations of the New Red pigments can also be used to reach the color spaces of existing, less sustainable, bright, yellow-shade reds that are currently available in the market.
For more information, visit www.bayferrox.com.















Report Abusive Comment