Materials that are safe, cheap and readily available are difficult to develop. The exponential growth in human civilization and steeply depleting natural resources are the key forces driving sustainable development. Changes in legislation and increased awareness among innovators and end users have motivated academia and industry to adopt safer chemicals and friendly routes for new developments. The paint and coating industrial sector is encouraged and facilitated from the steps taken by academia in training the next generation of scientists and engineers towards sustainable growth and the development of new technologies. A recent analysis revealed that the world’s petroleum feedstock may not last long, depending on future demand. The chemical industry is one of the leading users of petroleum feedstock for the production of essential commodities. Notably, the paint and coatings industry is the foremost player in using petroleum feedstocks for developing its broad range of products. It is therefore vital for our industry to initiate efforts in the direction of self-sustainability. An intricate journey leading to a sustainable route is briefly outlined in this article. This brief review cannot be treated as comprehensive information available in open and patented literature but rather an overview of recent developments in sustainable chemicals for the paint and coatings industry. Also, no attempt has been made to promote any particular technology or product with respect to their competitor.
The Pollution Prevention Act of 1990 laid the foundation for the Environmental Protection Agency (EPA) in 1991 to adopt the concept of “green chemistry”. Dr. Kenneth G. Hancock, Dr. Joseph Breen and Dr. Paul T. Anastas were among the first few advocates of the green chemistry revolution. The term “green” was probably coined by Jan S. Aubert in his article on environment-friendly coatings that appeared in 1993.1 Later in 1998, Paul Anastas and John Warner published a book that affirmed the 12 principles of green or environment-friendly chemistry. The term green chemistry has become a synonym for sustainable chemistry and has been used interchangeably. However, green chemistry can be treated as a subset of sustainable chemistry. Increased awareness and lucrative government incentives in developed countries have motivated a large group of companies to adopt sustainable routes of technological developments. Among the chemical industries, the coatings industry has been at the forefront in adopting sustainable chemistry routes. Several global companies like AkzoNobel, PPG, Henkel, Axalta, RPM, Valspar, BASF and 3M have taken initiatives for the sustainable transformation of their business. Both government and industrial sectors are investing significantly for the development of environment-friendly products that could meet the stringent requirements similar to currently available products. According to a recent market research report, the global market of environment-friendly coatings in 2012 was estimated at about USD $84 billion and is expected to reach approximately USD $117 billion in 2018. This growth at a CAGR of 5.6 is due to the high demand of automotive, architectural and packaging coatings, and the printing industry.
Environment-friendly (green) coatings are primarily classified as radiation-curing, water-based and high-solid materials. These are also sub-classified based on application, such as high-performance (corrosion protection), automotive (topcoat), architectural (decorative), wood (surface finish) and packaging (sealant). The progress of direct-to-metal UV-curable corrosion protection coatings is still in its infancy due to the numerous challenges in the development of such formulations. The adhesion of a coating to a metal surface, curing of thick coatings, a mismatch in coefficient of thermal expansion between coating and substrate while curing, excessive coating shrinkage, and service life span are a few of the unwanted hurdles facing formulators. Water-based and high-solid-content formulations are better appreciated as corrosion barrier coatings due to their low volatile organic compound (VOC) content. Radiation-curable coatings are gradually making their way into the automotive sector. The photo or radiation curing of these coatings in cracks/crevices, edges and corners is still a challenging task for developers due to limited access in these spaces. Interestingly, for such tedious jobs, scientists have devised innovative dual-curing coating technologies. The visible surfaces can be effectively cured with UV light while the hiding spaces self-cure in ambient conditions with time.
Global Regulations and Policy Decisions
Executive Order 13101 signed by President Clinton in 1998 led the foundation for the EPA to create a Framework for Responsible Environmental Decision Making (FRED). The FRED decision-making methodology enables the application of life-cycle assessment for comparing and analyzing competing products for their impact on human health and the environment. It also helps in the decision-making process for achieving a proper balance between the cost of a product, its technical performance and environmental issues. From an industrial point of view, the outcome of this order motivated companies to develop eco-friendly technologies and products. Moving several steps ahead, the European Union (EU) devised a mechanism of controlling toxic chemicals and harmful substances. The Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation, which was introduced in 2006 and enforced in June 2007, has brought the use of a significant number of chemicals under scrutiny. REACH has been considered the most complex legislation in the history of the EU that addresses the production, use and potential impact of chemical substances on human health and the environment. REACH mandates the registration of chemical substances at the European Chemical Agency that are either produced or imported in the EU and exceed the quantities of one tonne or more.
The Resource Conservation and Recovery Act (RCRA) of 1976 was developed by the EPA to address the ever-growing volume of waste from municipal and industrial sectors. RCRA has been referred as law, regulation and EPA policy and guidance that control hazardous waste from “cradle-to-grave”. This act not only regulates the generation, storage, transportation, treatment and disposal of hazardous waste but also establishes a framework for the management of nonhazardous solid wastes. With the introduction of the Toxic Substances Control Act (TSCA) in 1976, EPA got the authority to test, record, report and restrict the use of chemical substances. The TSCA was amended by President Barack Obama in June 2016 to the Frank R. Lautenberg Chemical Safety for 21st Century Act. This improved law facilitates the EPA in evaluating the existing chemicals on the basis of risk-based safety standards. Moreover, now the EPA has to agree before a new chemical can be introduced into the market. This will help users to identify and understand the nature of chemicals in household consumer products.
Material Safety Data Sheets (MSDS) from the Occupational Safety and Health Administration (OSHA) have been important documents generated by chemical manufacturers, and contain comprehensive information about the substance or mixture being used in workplace chemical management. The MSDS is a several-pages-long document that is shipped to users to warn them about the dangers related to the products, and provide them a guideline of safe handling, storage and disposal. The MSDS system was changed to the Safety Data Sheet (SDS) system in June 2015 to meet the requirements of the United Nations-adopted Globally Harmonized Systems (GHS) of Classification and Labeling of Chemicals. A tremendous amount of work was recently accomplished by chemical substance manufacturers in transforming the MSDS to SDS in order to comply with GHS. Noticeably, there could be major implications of the Britain Exit (i.e., Brexit) referendum 2016. Britain’s decision to move out of the EU will push chemical manufacturers to create a new database complying with the Control of Substances Hazardous to Health Regulations (COSHH) of 2002 in the United Kingdom.
Patent Search Report
A patent search of the terms “Environment Friendly” and “Coatings” resulted into 900 patent documents filed or published since 1997. To accomplish the patent search, the following International Patent Classification (IPC) filters were used: Class C09 (Main group - chemistry, metallurgy, dyes, paints, polishes, natural resins, adhesives, compositions not otherwise provided for, applications of materials not otherwise provided for); Subclass C09D (coating compositions e.g., paints, varnishes or lacquers, filling pastes, chemical paints or inks removers, inks, correction fluids, wood stains, paste or solid for coloring or printing of materials therefore); and Main group C09 D5/00 (coating compositions e.g., paints, varnishes or lacquer characterized by their physical nature or the effects produced, filling pastes). More specifically, to cover only certain types of environmentally friendly coatings related to corrosion, the following IPC filters were utilized: C09D5/02 (emulsion paints); C09D5/03 (powder coatings); C09D5/08 (anticorrosive paints); C09D5/14 (paints coatings biocides, e.g., fungicides, insecticides, pesticides); C090D5/16 (antifouling paints; underwater paints); C09D5/18 (fireproof paints) and C09D5/24 (electrically conductive paints). Similarly, the following Cooperative Patent Classification (CPC) filters were applied for the search: Main group C09D5/1637 (nanotechnology for materials or surface science); C09D5/002 (priming paints); C09D5/02 (emulsion paints including aerosols); C09D5/004 (reflecting paints, signal paints); C09D5/033 (characterized by additives); C09D5/082 (characterized by the anticorrosive pigments); C09D5/086 (organic or nonmacromolecular compounds); C09D5/1618 (inorganic compounds); C09D5/1668 (vinyl type polymers) and C09D5/1675 (polyorganosiloxane-containing compositions). The document collection search from USPTO, USAPP, EP, JP, WIPO, DE and Other Global were confined for the countries AU, EP, GB, KR, CA, FR, JP, US.
Figure 1 shows the distribution of the number of patents filed in the past 20 years, i.e., between 1997-2016. It is interesting to note that out of 900 patents filed, only 24 were filed before the executive order signed by President Clinton, a number that is much lower than the overall average of 45. However, there was almost a two-fold increase in the number of patents in the years 1999-2000, and a significant increase in filing was observed from 2001 onwards. There was a serious drop of about 58% in patent filing in 2004 compared to 2003, probably due to the rise in feedstock costs as a result of the increased price of natural gas and crude oil.2 There is a regular increment in the number of patent filing that peaked in 2013. A greater than 53% hike in patent filing was recorded in 2013 compared to the global average. Surprisingly, the number of patent filings dropped significantly in 2015, probably due to the slow recovery of the construction and consumer markets.
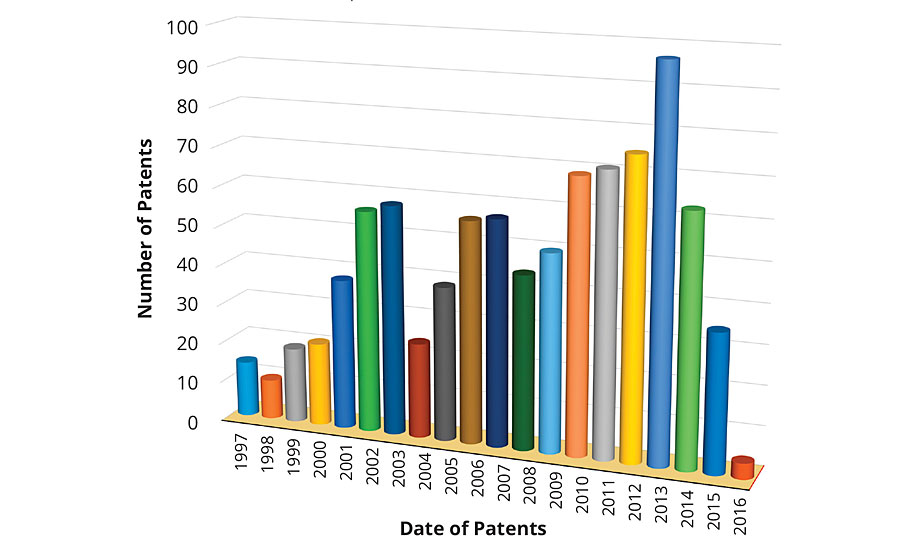
FIGURE 1 » Number of patents issued as a function of time.
Figure 2 shows the top 10 places that issued patents on environmentally friendly coatings in the past decade. The United States ranked first among the number of patents issued, followed by those that appeared as Patent Cooperation Treaty (PCT). An approximate equal number of patents were issued in China and Europe. The number of patents issued by the United States was more than two times any other country in the world. This clearly demonstrates the commitment and investment toward sustainable development. Looking to the patent technology evolution map from the top 20 companies in Figure 3, it is apparent that the first five companies, i.e., 3M, Chemetall, Evonik, AkzoNobel and Boeing, have received an almost equal number of patents in the past 10 years. Since the numbers of patents filed in 2013 was exceptionally high, this resulted in a dense crowding of balloons in 2014 and 2015. Moreover, it is interesting to record that the evolution of sustainable technologies has been consistent among the five mentioned companies. The eco-friendly technology to develop an antifouling underwater paint/coating gained maximum attraction, followed by anticorrosive coatings/paints and emulsion paints. Technology to develop biocidal coatings is among the fastest-growing area of research and development, followed by powder coating products.
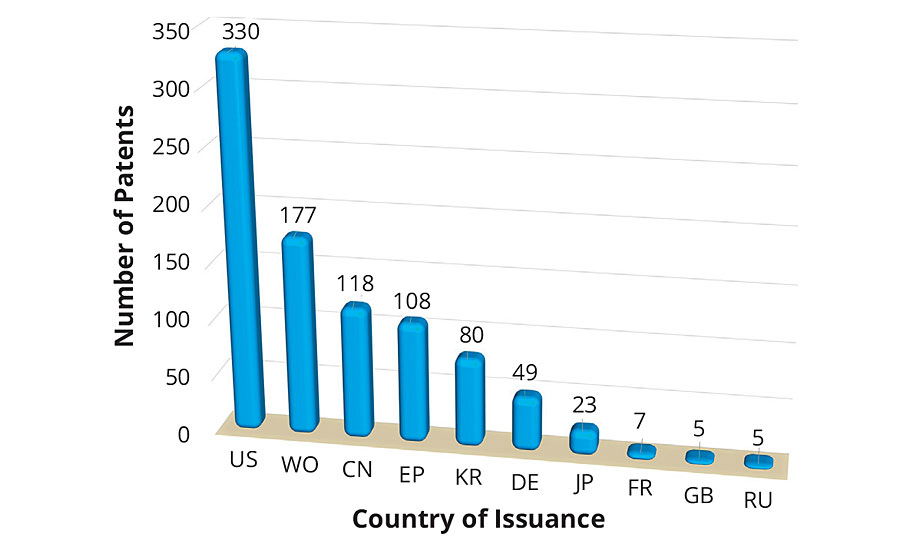
FIGURE 2 » Number of patents issued to countries.
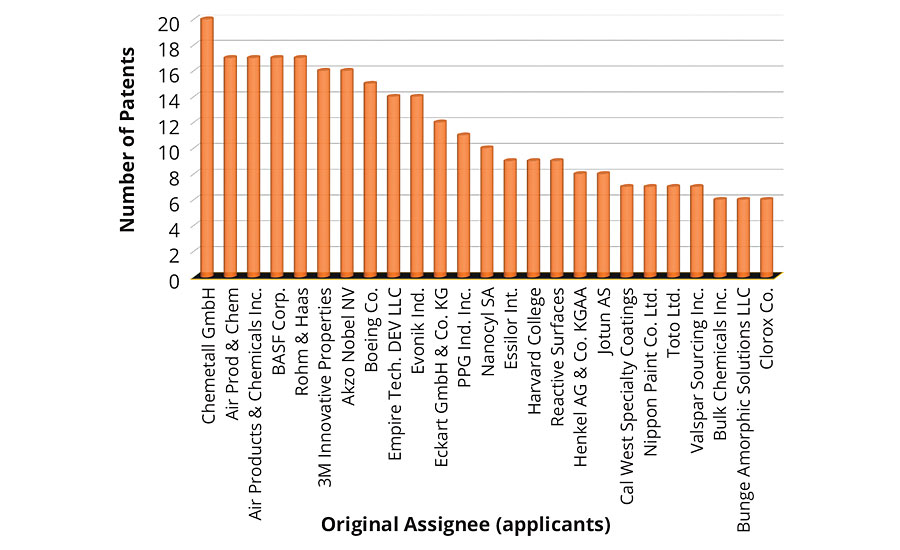
FIGURE 3 » Number of patents issued to individual top 20 assignees.
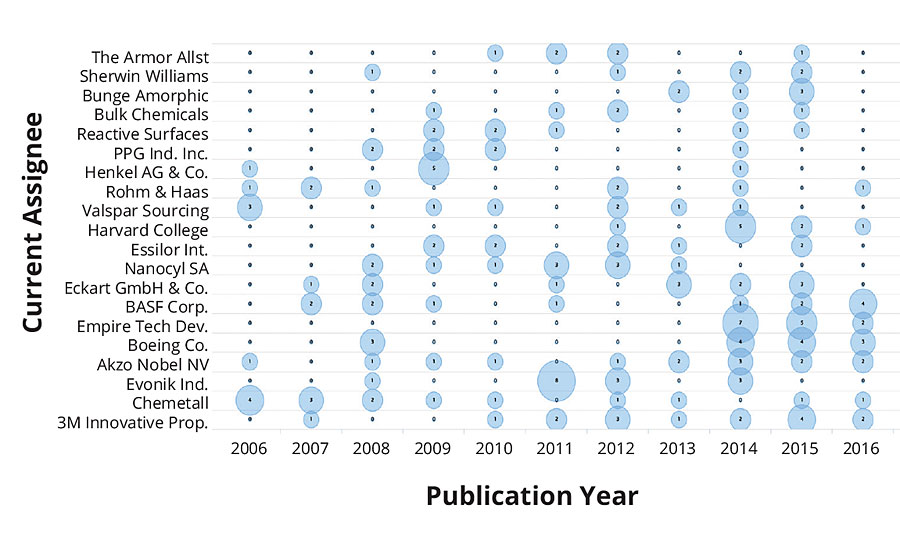
FIGURE 4 » Evolution map of technologies in top 20 companies.
Current State of Research and Development
A Scopus search of the term “Green Chemistry” resulted into 36,275 articles that were published between 2006-2016. This means that on average, more than 3,600 articles were published each year, or about 15 articles every working day in the last 10 years. Contrary, the search term “Environmentally Friendly Coatings” resulted in just 611 articles, and the terms “Coatings” and “Green Chemistry” resulted into 576 articles in the last 10 years. Figure 5 shows the distribution of articles on green chemistry.
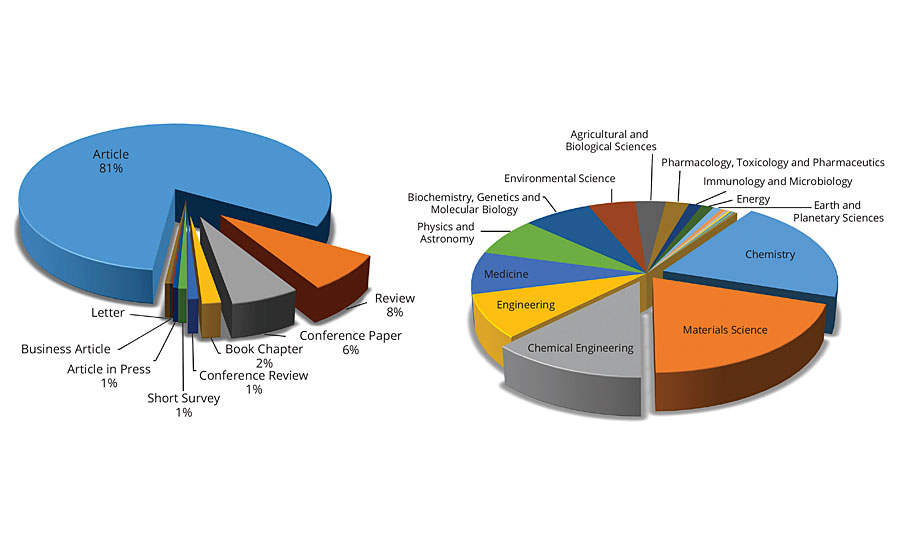
FIGURE 5 » Distribution of articles related to environmentally friendly coatings.
The first few reports on the development of environmentally friendly coatings appeared in the early 90’s.3 In 1993, the United States Department of Agriculture published a situation and outlook report describing the applications and investment in plant-based materials.4 In the same year, Bayer Inc. introduced its low-viscosity, environment-friendly polyurethane system based on the aromatic isocyanate (Desmdour VL) and polyol (Desmophen 1150).5 Consequently, several articles related to environmentally friendly coatings appeared in mid-1990. Derksen et al.6 published a review article on the renewal resources in coating technologies that described the possible use of a wide variety of vegetable oil-based binders. His review highlighted that the durability and hydrophobicity in the coating can be modified by varying the degree of crosslinking in the protein binders.
The commercial electrocoat technologies that evolved in the early 1960s started using environment-friendly and economically efficient solutions. Most of the automotive industry and Original Equipment Manufacturers (OEMs) started adopting electrocoat technology due to its enormous benefits, including total uniform coverage of complex parts, high material transfer efficiency, low VOCs and water emission, safe and clean operating conditions, etc.7 Zinc-rich epoxy coatings also gained attention for those metals that were hard to coat with electrocoat technology. As an example, a successful combination of sacrificial zinc-rich primer, epoxy resin primer and polyurethane topcoat was applied on the Kansai International Airport Bridge, Tokyo Bay Crossing Bridge and world’s largest cable stayed bridge, Akashi Kaikyo Ohashi, in Japan.8 Besides metal-filled coatings, the conducting polyaniline coatings were also investigated as an environmentally friendly route to anticorrosion coating. However, the effectiveness of conductive polymer coatings at variable pHs always remained in question.9 The dense High Velocity Oxygen Fuel (HVOF)-sprayed face coating appeared in 1997 and made its way in the industrial sector. HVOF coatings were considered an excellent replacement of chromate plating and contained reasonable crack resistance in their service life span.10
Most of the new coating systems being tested as a replacement for chromate conversion coatings (CCC) contained high VOCs that were considered responsible for global warming, as well as the formation of smog, leading to Organs Psycho Syndrome. The European community started looking into controlling or limiting the emission of VOCs through the COM(96) 538/4 Council Directive. Following the initiatives from the European community, the coatings industry started transitioning into high-solid coating compounds, powder coatings and water-based acrylics or alkyd emulsions. The high-solid alkyds suddenly turned into an industry favorite due to associated benefits such as thicker layer application with enhanced hiding power, resulting in much lower labor expenses with the use of standard equipment and knowledge inherited over time. To mitigate the effects and vacuum left as an eminent retirement of CCC, several chromium-free (or nonchrome) pretreatments were investigated on different metal alloys. However, none could meet the gold standards established by CCC until Pantheon Chemicals introduced PreKote®, a nonchrome surface pretreatment that gained significant attention in late 90’s. PreKote appeared as a disruptive technology for numerous nonchrome primer coating systems in the aviation industry and is still considered a benchmark solution to mitigate corrosion in metal alloys.11
The low-friction, diamond-like carbon (DLC) coating made through the physical vapor deposition technique was also tested in automotive components and gained significant commercial success. The doping of tungsten carbide in DLC gave industry an option to coat metal parts with a durable coating having high elasticity and low coefficient of friction, along with significantly high hardness and chemical resistance.12 The projected global DLC coating market reached US$1.7 billion in 2015. Although the phosphate conversion coating proved to be extremely effective in preventing steel substrates from corrosion it is not discussed here due to toxicity involved with the use of chemicals and the process. Contrary, the safer conversion coatings based on rare earth metals including cerium and lanthanide compounds gained appreciable attention as an effective barrier to corrosion.13 The scarcity of rare earth metals and limited corrosion prevention checked the wide-scale adaptability of such coatings.
The prevention through passivation of metal alloys using sol-gel-derived materials also gained remarkable attention. A remarkable work accomplished by Rustom Roy, Jeffrey Brinker and George Scherer led the foundation of numerous sol-gel technologies.14 Following their work, an extensive use of silicones, titanium, zirconium, cerium and alumina compounds was realized in coating compositions.15 Tin compounds such as dibutyltindilaurate and tributyltin have been extensively used not only as catalysts for ambient curing of coatings but also as biocides in antifouling paints and coatings. Biofouling on the surfaces of ocean vessels is considered a critical problem and challenge for coating scientists (Figure 6). Industry has been actively looking for a coating composition that can prevent the adhesion of invasive mussels and barnacles to the hulls of ocean vessels.16,17 Another thermal diffusion coating process using thermo-chemical surface modification (TCSM) gained reputation in industry, as the process was environment friendly and does not involve any harmful substances. The metal surface could be improved through TCSM by modifying the characteristics of intermetallics that are vulnerable to corrosion attack.18

FIGURE 6 » Severe biofouling activity on marine vessel. This picture first appeared on July 4th, 2011 in The Economist.
Chemical agent-resistant coating (CARC) systems have been popular in the coating of U.S. military vehicles, as they provide long-term protection from chemical and biological agents (as per MIL-DTL-53072E). The original CARC system was made up of high-VOC chromate wash primers (as per DOD-P-15328 and MIL-C-8514C), a high-VOC, chrome-free epoxy primer (as per MIL-P-53022) and high-VOC two-component polyurethane topcoat. It was discovered that the use of hexamethylene diisocyanate (HDI) used in the curing of polyurethane resins was hazardous to human health. HDI poses a significant health threat due to inhalation during sanding, grinding or any other conditions that could produce CARC fumes, dust or vapors.19 The Army Research Lab motivated suppliers to reduce the VOC content (as per MIL-DTL-53039 Type II specification) and eliminate the Hazardous Air Pollutants (HAPs) from CARC formulations (as per MIL-C-53039). The original CARC system (MIL-C-46168) phased out in late 2005 after water-dispersible, HAPS-free and low-VOC topcoats became available. A new MIL-DTL-53030 that contained a low-VOC and HAPs-free water-reducible epoxy primer has been used with MIL-DTL-64159 Type II CARC topcoats to achieve optimum corrosion protection.19
The bluing of steel substrates, especially gun bluing, has been considered one of the crucial electrochemical conversion coating technologies for steel, cast iron and stainless steel parts. This process converts iron into Fe3O4, but is not effective on aluminum and other metal alloys. The polytetrafluoroethylene (PTFE)-doped inorganic coating using electroless or electrolytic methods was also adopted for the coating of metal substrates. The uniform, nodular dispersion of PTFE provided good corrosion and fouling protection to metal surfaces.20,21 Several other variations of PTFE-doped nickel coatings were developed later that are still used commercially. Both the nickel and PTFE were later found harmful for human and environmental health, and coatings made out of these are not truly green.
Direct-to-metal (DTM) coatings have been considered as a replacement of the two-layer primer and topcoat system. DTM coating technology appealed to OEMs, and a wide variety of formulations appeared on the market within a short span of time. Aliphatic polyurea-based polyaspartic coatings and UV/EB-cured polyacrylate coatings have gained good momentum in the past few years. The DTM use of UV/EB-cured coatings is an area of extensive research and development. The availability of a wide range of photoinitiators and efficient curing techniques is propelling the easy adaptability of these coatings in the industrial sector.22 Among other polymer coatings, a green hybrid polyurethane coating has recently been developed without the use of toxic isocyanates.
Conclusion
Although enormous efforts have been made to find sustainable solutions for making commercially successful products, the quest for environment-friendly coatings is still in its infancy. The new health and safety regulations imposed on chemicals and coating manufacturing will certainly motivate industry in the direction of sustainable development. Although the slow recovery of the worldwide economy has severely affected the ongoing efforts to sustainable research and development, the pace of innovation and invention has not been affected. The increased awareness by the consumer and exceedingly high demands for safer products have posed a challenge for researchers to meet these expectations. A significant amount of funds and resources are being allocated by companies and governments toward achieving sustainability in consumer product developments.
Acknowledgement
I would like to thank Dr. Peter Zarras from the Naval Air Warfare Center Weapons Division, Polymer Science & Engineering Branch, California, USA, for suggesting modifications. The patent analysis was conducted using the evaluation version of AcclaimIP and Scopus search engines. I also gratefully acknowledge Anaqua Inc. and RELX Group for providing the evaluation version of these tools.
References
1 Aubert, J.S. Anti-Corrosion Methods and Materials, Vol. 40, 15-16, 1993.
2 Tullo, A.H. Industrial Coatings Market is Beginning to Recover as Companies Mull New Markets and Technologies, Chemical & Engineering News, American Chemical Society, pp. 25-31, 2004.
3 Lowrance, R.E.; Paul, K.P. Environment-Friendly Alternatives for the Formulation of VOC-Compliant Silicone Coatings, Proceedings of the Water-Borne and Higher-Solids Coatings Symposium, Univ of Southern Mississippi, Univ of Southern Mississippi, 1990.
4 Curran, M.A. Environmental Progress, Vol. 22, 277-292, 2003.
5 Anon, Anti-Corrosion Methods Mater., Vol. 40, 1993.
6 Derksen, J.T.P.; Cuperus, F.P.; Kolster, P. Ind. Crops Prod., Vol. 3, 225-236, 1995.
7 Oravitz, J.J. Prod Finish (Cincinnati), Vol. 60, 48-51, 1996.
8 Kawanishi, H. Zairyo Kankyo, Vol. 47, 691-695, 1998.
9 Racicot, R.J.; Yang, S.C.; Brown, R. MRS Online Proceedings Library Archive, Vol. 488, 733 (738 pages), 1997.
10 Rastegar, F.; Richardson, D.E. Surface and Coatings Technology, Vol. 90, 156-163, 1997.
11 Roberts, L.; Galanis, A. Paint and Coatings Industry, Vol. 19, 86, 2003.
12 Gåhlin, R.; Larsson, M.; Hedenqvist, P. Wear, Vol. 249, 302-309, 2001.
13 Aballe,A.; Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M. Mater. Corros., Vol. 53, 176-184, 2002.
14 Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st Edition ed., Academic Press, 1990.
15 Metroke, T.L.; Parkhill, R.L.; Knobbe, E.T. Progress in Organic Coatings, Vol. 41, 233-238, 2001.
16 Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Progress in Organic Coatings, Vol. 50, 75-104, 2004.
17 Almeida, E.; Diamantino, T.C.; and de Sousa, O. Progress in Organic Coatings, Vol. 59, 2-20, 2007.
18 Fearheiley, P. Finishing Today, Vol. 84, 18-19, 2008.
19 Arent, G. Finishing Today, Vol. 84, 26-27, 2008.
20 Pena-Munoz, E.; Berçot, P.; Grosjean, A.; Rezrazi, M.; Pagetti, J. Surface and Coatings Technology, Vol. 107, 85-93, 1998.
21 Zhao, Q.; Liu, Y.; Müller-Steinhagen, H.; Liu, G. Surface and Coatings Technology, Vol. 155, 279-284, 2002.
22 Tiwari, A.; Polykarpov, A. Photocured Materials, Smart Materials Series, Royal Society of Chemistry, London, pp. 370, 2014.





Report Abusive Comment