It is well known that utilizing a redox pair is an effective way to reduce free monomer at the end of an emulsion polymerization process. This is because a redox couple generates a large amount of free radicals capable of converting low levels of free monomer. The ability to use redox chemistry for initiation of main polymerization is also known to be effective. For some systems, these redox reactions can be done at or below room temperature.
A new family of advanced sulfinic acid derivative reducing agents has been developed that offer higher reactivity when compared to conventional reducing agents in the post polymer-ization. This work was undertaken to determine if they could be used for the initiation of main polymer-ization, and what impact they would have on polymer properties, performance and monomer conversion.
This article will present results comparing thermal initiation of main polymerization to redox initiation at lower temperatures. Two series of tests were performed – one using a 45%-solids latex, the second using a 50%-solids latex.
For each series, acrylic latex of butyl acrylate, methyl methacrylate and methacrylic acid was produced. Data was developed comparing a thermally initiated latex to one using redox for the main polymerization. A redox chase was then performed for each case. Residual monomer levels and polymer properties are shown to compare the performance of each test.
Main and Post-Emulsion Polymerization
Many current emulsion polymer production recipes use a combination of thermal and redox radical generation, in two distinct steps in the process, main and post polymerization.
A typical main polymerization process utilizes a persulfate to generate radicals, initiating and sustaining polymerization. Heating the emulsion with the persulfate initiator to ~ 80 °C cleaves the persulfate molecule into two radicals. Conversion of the monomer continues, however the curve of monomer conversion is asymptotic, resulting in a very long time for little monomer conversion at the end of the main polymerization. Although this results in 99% + conversion of the monomer, monomers used in emulsion polymerization are VOCs and typically have an offensive odor.
Post polymerization using redox chemistry is currently utilized for most emulsion polymers. When introduced into the system toward the end of monomer conversion in the main polymerization period, a flood of radicals is introduced into the system, converting monomers into dimers, oligomers and short polymer chains. Since most of the monomer has already been converted into desired polymer in the main polymerization step, these oligomers are not viewed as detrimental to the formed polymer. It can be viewed as a “clean up” operation to reduce free monomer, resulting in lower VOCs and odor.
Redox components used in production post polymerizations typically use the following:
Oxidizers: Hydrogen peroxide
tert-Butyl hydroperoxide (tBHP)
Ammonium persulfate (AP)
Reducing agents: Ascorbic acid (AA)
Sodium formaldehyde sulfoxylate (SFS)
Sodium sulfite
Sodium metabisulfite (SMBS)
Advanced sulfinic acid derivative
Comparison of Various Reducing Agents for Redox-Initiated Main Polymerization
An earlier study by BrüggemannChemical screened several reducing agents to determine which one would produce the fastest and most complete conversion of monomer in a styrene-butyl acrylate system.
The testing utilized an adiabatic system to correlate temperature rise with monomer conversion. Tests were conducted with and without the addition of an iron sulfate heptahydrate solution.
This reaction mixture was heated to 60 °C. Shots of the redox couple (selected reducing agent + ammonium persulfate) were added initially and at the 60/120/180-min marks. Both reducing agent and ammonium persulfate solution concentrations were 56.24 g/L. Each shot consisted of 10 mL of this solution, added simultaneously via separate feed lines.
In both cases, an advanced sulfinic acid derivative showed the earliest and highest temperature rise, indicating faster and more complete conversion of monomer, with or without addition of an iron transition metal. Virtually no temperature rise was observed with the advanced sulfinic acid derivative with subsequent addition, with or without iron sulfate heptahydrate solution. This indicates full conversion with only one dose of the advanced sulfinic acid reducing agent, showing it to be far more effective than the others tested.
The other reducing agents showed far less activity, and needed three or four doses of the redox couple to start polymerization and begin generating heat. In this test, Figure 1 reveals that an advanced sulfinic acid derivative is much better at converting monomers than the other three reducing agents tested.
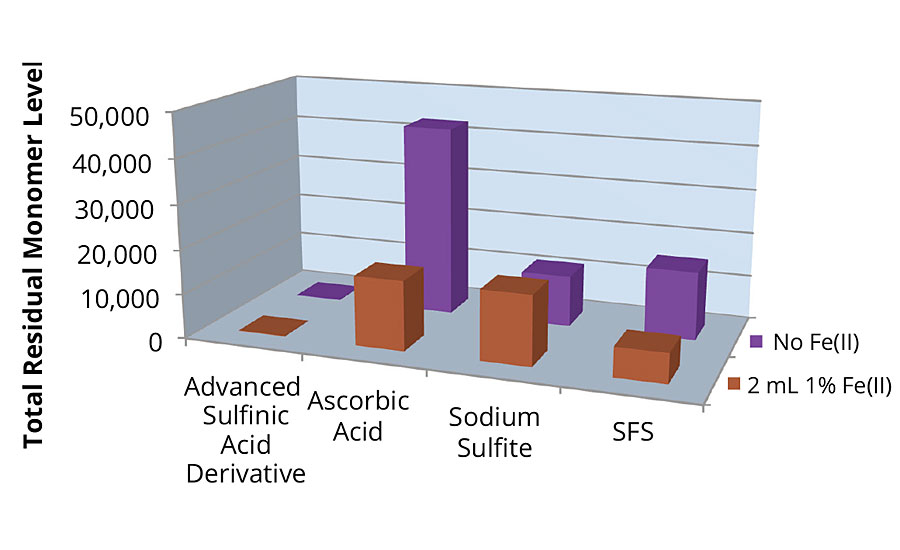
FIGURE 1 » Residual monomer level of reducing agent tests.
Further Study of Thermal vs. Redox for Main Polymerization
The results of the earlier work from Figure 1 prompted further study of the use of a redox couple to initiate main polymerization in a new all-acrylic system. These tests utilized standard industrial equipment, practices and materials that are common to most emulsion polymer facilities.
All tests were performed in a 3 L glass-jacketed reactor fitted with a four-neck top. Inserted into the necks were inlets for oxidizer, reducer, stirrer, nitrogen, thermocouple, monomer emulsion feed tube and condenser. During polymerization, samples were analyzed for residual monomers to measure effectiveness over time. Physical properties of the final polymer were compared.
Two series of tests were performed, the first with a 45%-solids latex, the second with a 50%-solids latex. The process approach utilized a seed polymer and a monomer pre-emulsion. In both series, for main polymerization, thermal initiation run at 80 °C is compared to 40 °C redox initiation. In the second series, a third case was run with redox initiation at 20 °C. These lower-temperature redox reactions were allowed to heat up adiabatically to increase reaction rate, negating the need for vessel preheating. Identical monomer feed profiles were used in each case. For all cases, after the main reaction step, an identical post polymerization step was performed to reduce residual monomers.
Series 1 Emulsions: 45%-Solids Latex
Monomer Emulsion Feed
Deionized water and sodium lauryl sulfate (SLS) at 29.5% solids were added to a flask and mixed for 1 min. Under agitation, to this flask was added in the following order; butyl acrylate, methyl methacrylate and methacrylic acid. These monomer levels were chosen to produce a latex with a glass transition temperature (Tg) of about 1 °C, common for latexes that are used in low- or zero-VOC paint formulations.
Reactor Charge
Water was added to a separate flask for the initial charge. Under agitation, to this flask was also added sodium bicarbonate, SLS and seed latex (previously prepared) to form the initial reactor charge. The seed latex amount was chosen to obtain the appropriate particle size at the end of the reaction and also to reduce batch-to-batch variation in particle size.
Oxidizer and Reducer Charges
The oxidizer and reducer solutions were made by dissolving the appropriate amount of each ingredient in water. They were made up just before the reactor contents reached the starting temperature of the reaction.
Reaction Procedure
The initial charge was added to the reactor. The headspace was purged rapidly with nitrogen for 1 min to eliminate oxygen. The reactor contents were then heated to the starting temperature. When the reactor charge reached the reaction set point, the oxidizer charge was added to the reactor. Simultaneously, the monomer pre-emulsion was started at the prescribed rate using a piston pump. After 30 min, the monomer pre-emulsion feed rate was increased to the new set point. The overall feed time for the monomers was 4 hrs. The oxidizer and the reducer (where appropriate) were started simultaneously with the monomer feed. These were fed at a constant rate, using a syringe pump. When the reducer was not used in this feed, water allocated for the reducer was still fed into the reactor to maintain consistent solids concentration for all runs.
For the 80 °C thermal initiation, the vessel was preheated to 80 °C. Only ammonium persulfate (APS) was used during the monomer feed (no advanced sulfinic acid derivative) since the temperature of the reaction allowed for an appropriate flux of APS radicals at 80 °C via the reaction [O3SO–OSO3]2− ⇌ 2 [SO4]•−.
After the 4-hr monomer pre-emulsion feed was complete, the reactor contents were cooled to 70 °C over 30 min to simulate transfer to a cooling tank. The redox of tBHP and advanced sulfinic acid derivative was then fed over 60 min. Simultaneously the reactor was further cooled to 60 °C. After this, the reaction was cooled to 20 °C.
The 40 °C redox initiation was preheated to 40 °C and allowed to heat adiabatically to 60 °C during the reaction. The advanced sulfinic acid derivative reducer was used to activate the APS at this low temperature. In an effort to simulate adiabatic temperature rise in the reactor, similar to industrial reactors, the glass reactor was insulated with packing cloth and covered with thick aluminum foil. Upon the start of the reaction, the heating water in the jacketed reactor was drained and the reactor contents were allowed to increase in temperature solely due to the heat of the reaction. The reaction temperature continued to increase throughout the monomer pre-emulsion feed. It reached a maximum of 60 °C at the end of the feed.
After the 4-hr monomer pre-emulsion feed was complete, the reactor contents were maintained at 60 °C for 30 min. Then the redox of tBHP and advanced sulfinic acid derivative was fed over 60 min while the reactor temperature was maintained at 60 °C. After this, the reaction was cooled to 20 °C. Further details on feed profiles are in Table 1.
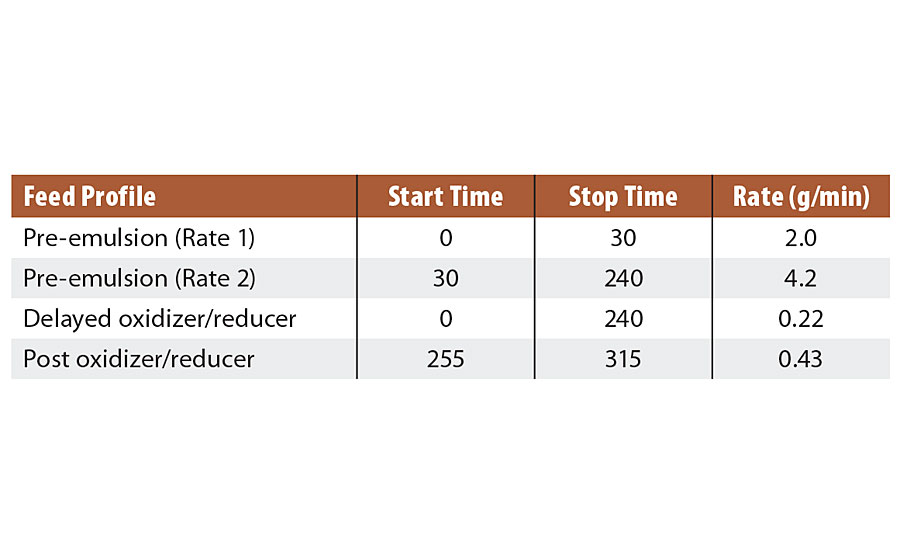
TABLE 1 » Feed profile for Series 1 emulsions.
Results
The results were similar for both reactions in most properties. Table 2 describes observations of the latex properties produced by both recipes used in the Series 1 tests.
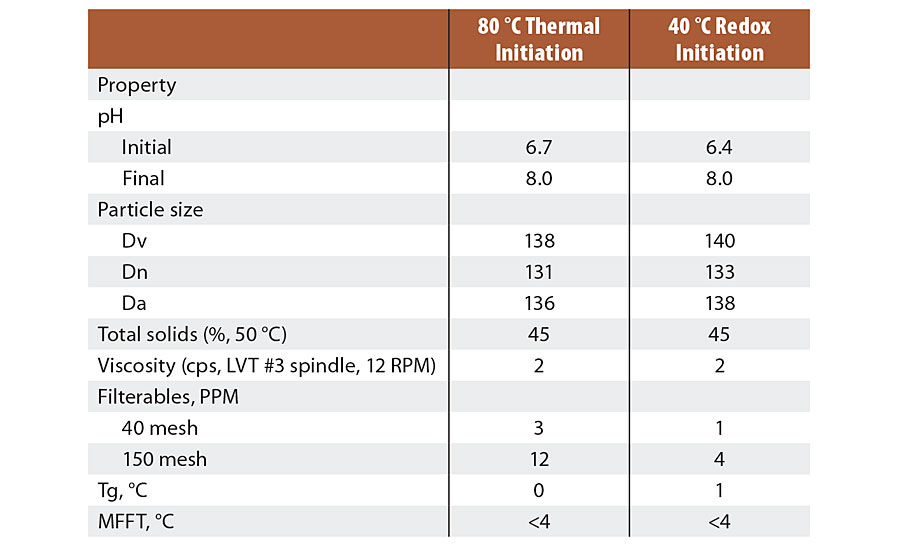
TABLE 2 » Latex properties for Series 1 emulsions.
Monomer Level Profile During Reactions
Samples for monomer levels were taken at four times during the reaction per the following:
- End of pre-emulsion feed.
- After 30-min hold to simulate transfer of the reactor contents to a post reaction tank. This often occurs in industrial processes. This is also the start of post redox feed.
- Middle of redox feed.
- End of redox feed.
As shown in Figure 2 and Figure 3, the reaction run with redox initiation in the main feed produced lower total levels of monomers than the thermal initiation. This is surprising since the redox reaction was performed at lower temperatures. Lower temperatures usually result in slower polymer growth. In both cases the post redox with tBHP and advanced sulfinic acid derivative was very effective in reducing total monomer concentration in the reaction.
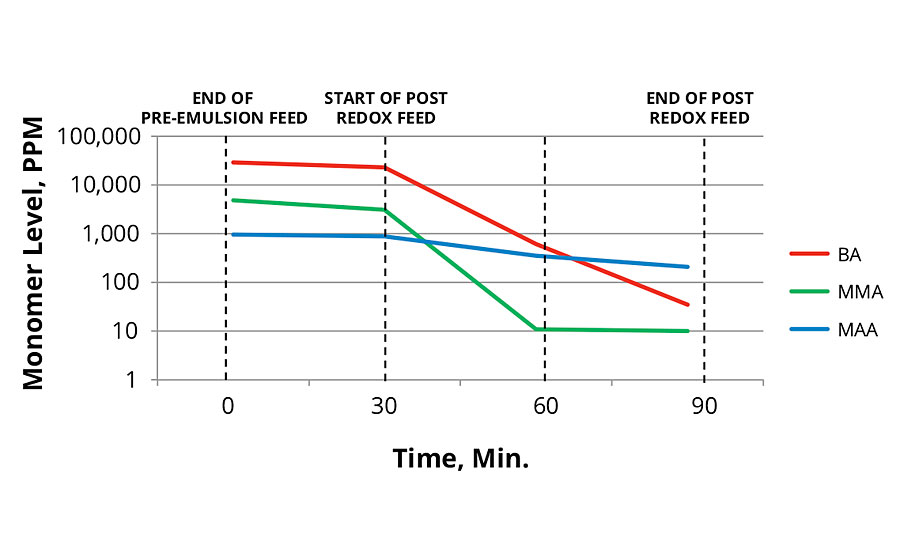
FIGURE 2 » Monomer levels of 80 °C thermal initiation.
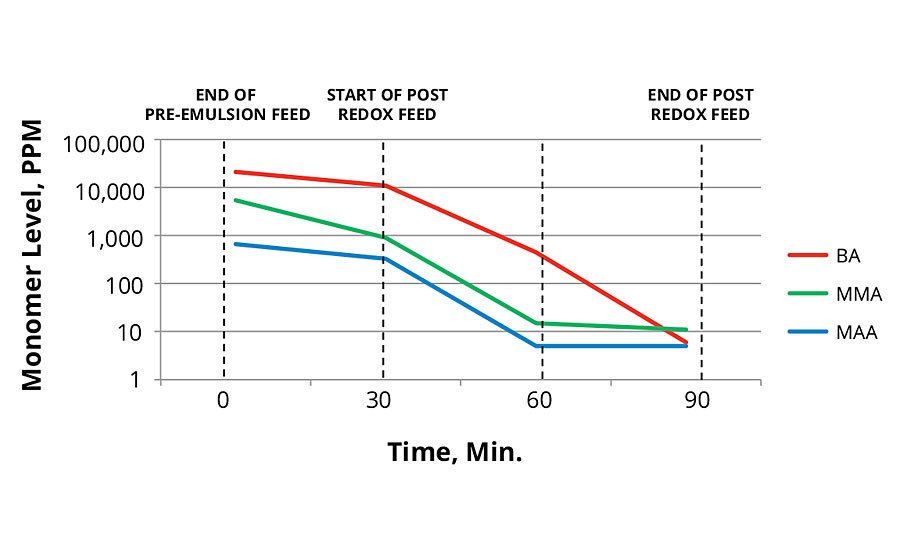
FIGURE 3 » Monomer levels of 40 °C redox initiation.
Molecular Weight Analysis
Each of the latexes produced in Series 1 were analyzed for molecular weight. HPLC conditions for molecular weight analysis were:
- Agilent 1100 system with RI detector.
- Column PLGel mixed D 7.5x300 mm + PLGel mixed C guard 7.5x50 mm.
- Column Temperature: 60 °C.
- Mobile phase: THF.
- Flow rate: 1 mL/min, isocratic.
- 40 µL injection.
Both reactions produced similar molecular weight polymers (Peak 1). Second peak (Peak 2) is a combination of oligomers and surfactant. Table 3 shows the molecular weight analysis results.
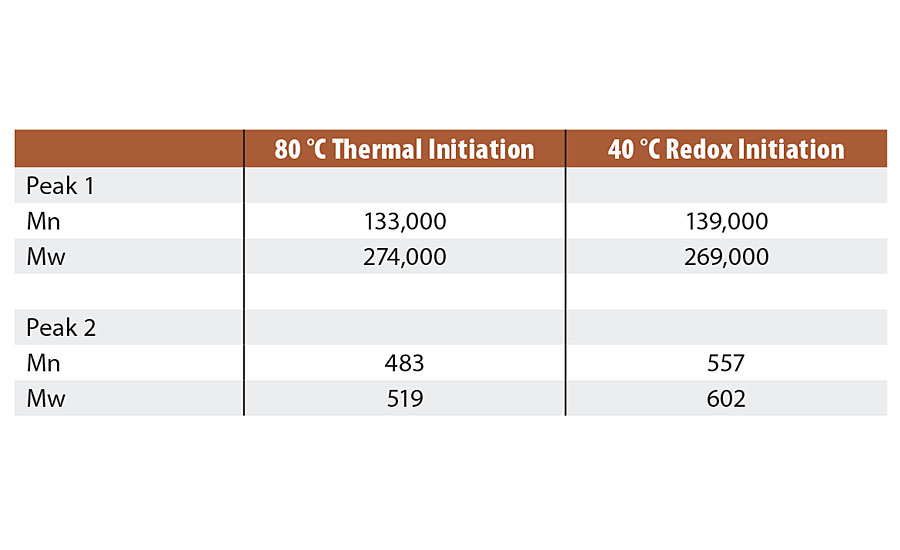
TABLE 3 » Molecular weight of Series 1 emulsions.
Gel Content Analysis
Dried latex specimens were weighed, placed in a 150 mesh stainless steel pouch and then immersed in extracting solvent (THF) for 3 days. After extraction, the specimens were removed, dried and reweighed and the gel content was calculated. The gel contents were similar between the two reactions, shown in Table 4.
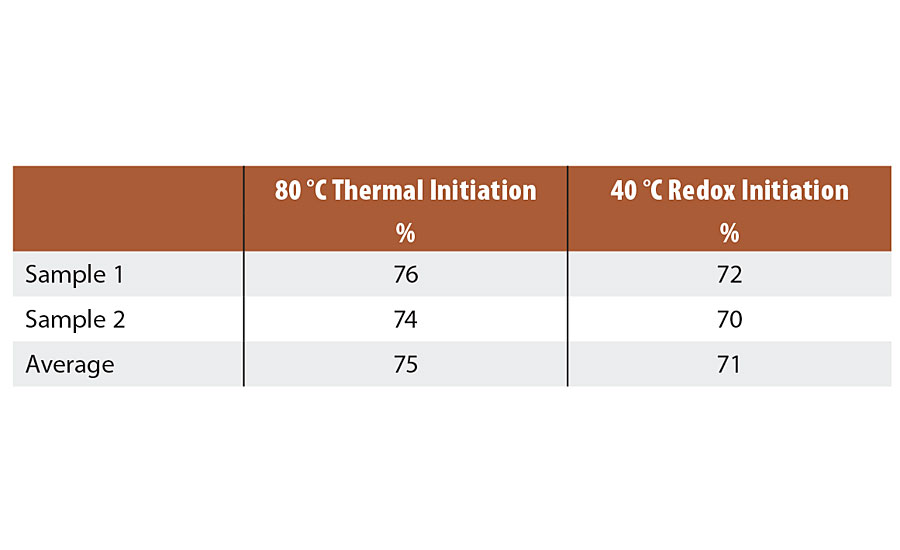
TABLE 4 » Gel content of Series 1 emulsions.
Series 2: 50%-Solids Latex
Reaction Procedure
All procedures were the same as in Series 1, except that tests were performed starting at 40 °C and 20 °C. Feed profiles used are listed in Table 5.
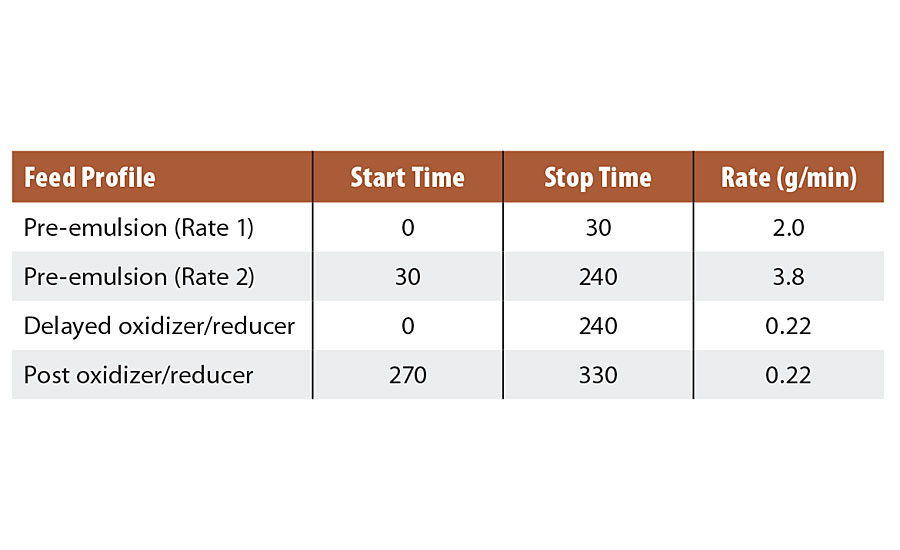
TABLE 5 » Feed profile for Series 2 emulsions.
Note that the data presented in this series shows progression of temperature and residual monomer levels over the entire reaction. This is different than the data presented in the Series 1 reactions, which show only the variables after the end of pre-emulsion feed.
Results
Figure 4 shows a profile of temperature for all recipes. Note the temperature rise for the redox-initiated latexes as the reaction heats the vessel adiabatically. During the first test with the 20 °C redox initiation, some evidence of monomer pooling was observed, as can be seen in the flat spots of the temperature curve. To eliminate monomer pooling, additional reducer was used in the first 30 min. Amount of reducer added during this time was doubled, an increase of 0.1125 grams, for a total amount of reducer in the main polymerization of 1.0125 grams. On a percentage basis, the amount of reducer went from 0.16% on monomer solids to 0.17%. Weight ratio of oxidizer to reducer went from 3.88/1 to 3.46/1
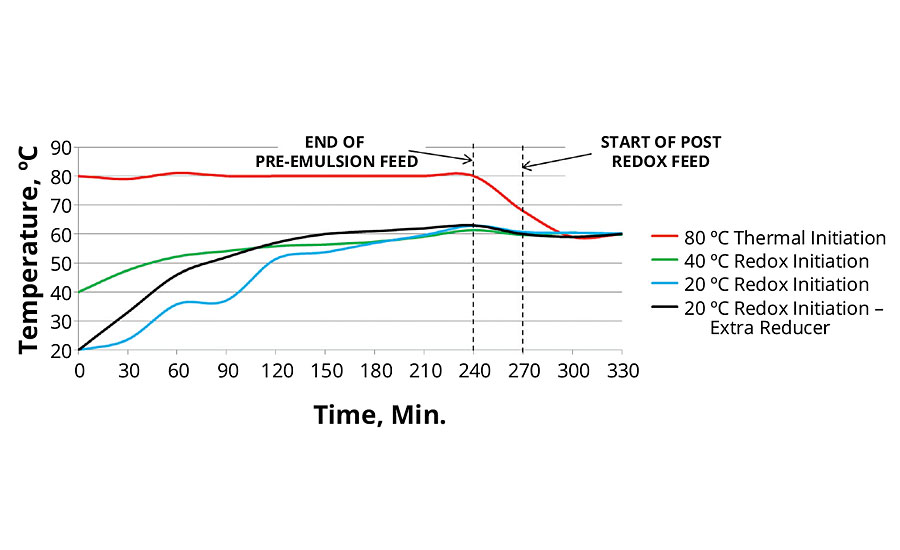
FIGURE 4 » Temperature profile of thermal and redox initiation.
Monomer Level Profile During Reactions
Samples for monomer levels were taken 11 times during the reaction:
- Every 30 min during monomer pre-emulsion feed (7 samples).
- End of pre-emulsion feed.
- After 30-min hold to simulate transfer of the reactor contents to a post reaction tank. This often occurs in industrial processes. This is also the start of post redox feed.
- Middle of redox feed.
- End of redox feed.
The 40 °C redox initiation reaction produced lower total levels of monomers than the 80 °C thermal initiation reaction. This is surprising since the redox reaction was performed at lower temperatures. Lower temperatures usually result in slower polymer growth. The 20 °C redox initiation produced slightly higher residual monomers, however when extra reducer was used, it resulted in the lowest total free monomer level.
In all cases the post redox with tBHP and advanced sulfinic acid derivative was very effective in reducing total monomer concentration in the reaction. Data for these reactions are presented in Figures 5, 6 and 7.
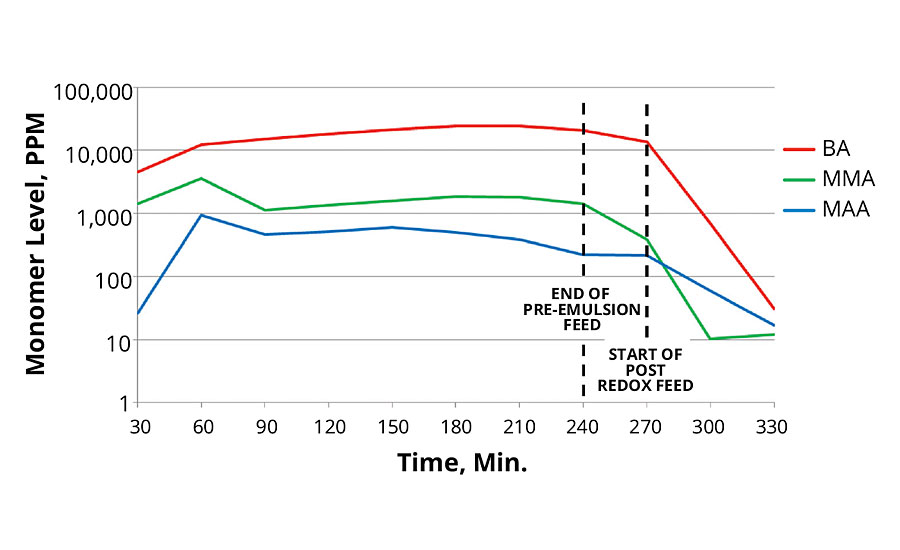
FIGURE 5 » Monomer levels of 80 °C thermal initiation reaction.
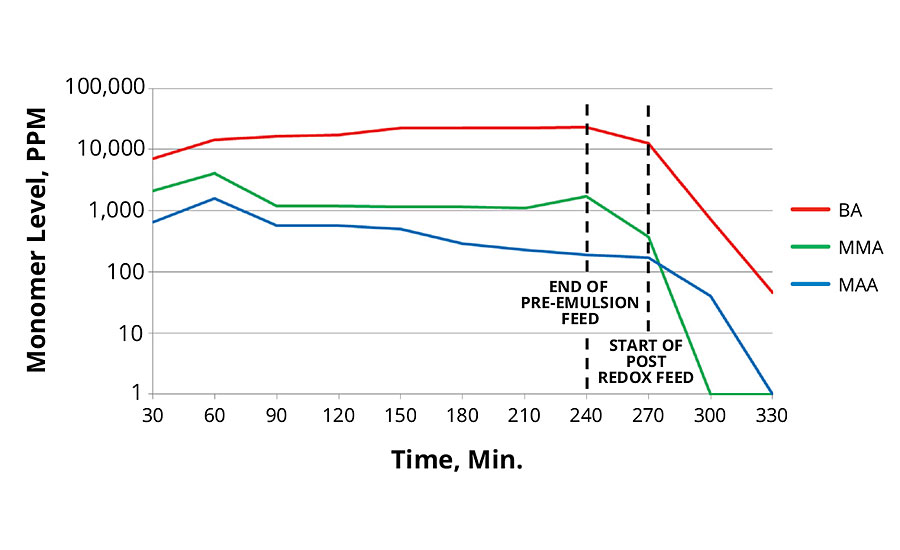
FIGURE 6 » Monomer levels of 40 °C redox initiation.
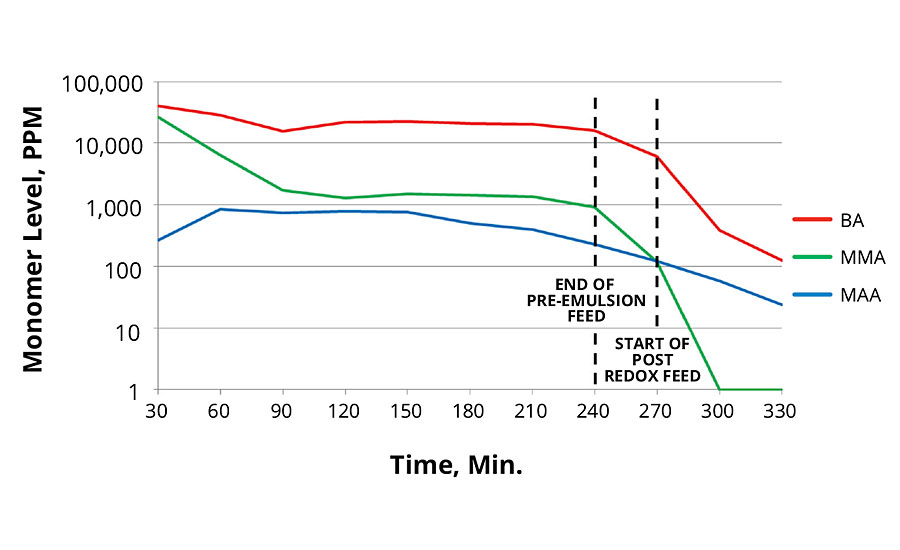
FIGURE 7 » Monomer levels of 20 °C redox initiation.
Combining the data to look at total free monomer for this series, and including the run with extra reducer for the 20 °C redox-initiated case, it is shown in Figure 8 that the redox-initiated main polymerization shows a significant reduction in total free monomer level.
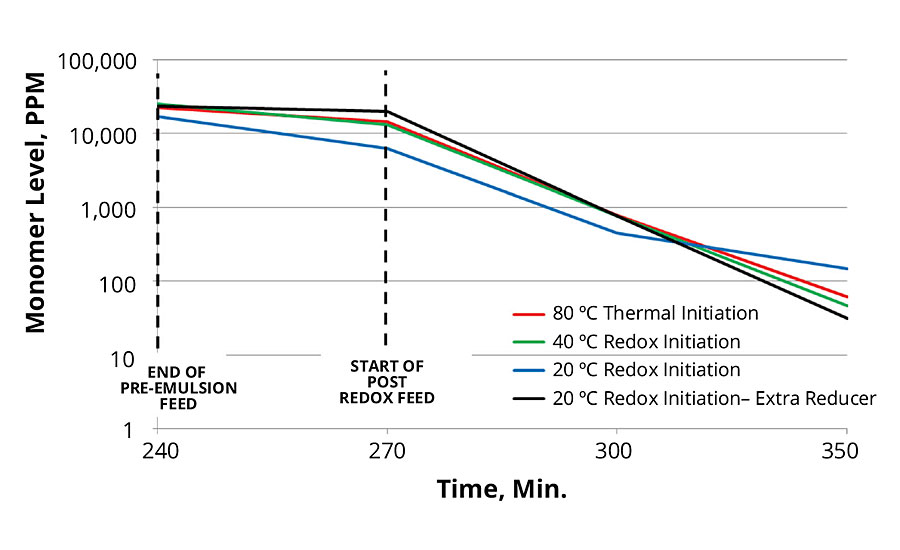
FIGURE 8 » Total free monomer comparison.
Latex Property Results
The results were similar for all three reactions in most properties. The main differences were seen in slightly larger particle size and the lower dry film gloss for the latex started at 20 °C. It should be noted that the filterable solids are very low and the reaction vessels were easily cleaned. Table 6 describes observations of the latex properties produced by both recipes used in the Series 2 tests.
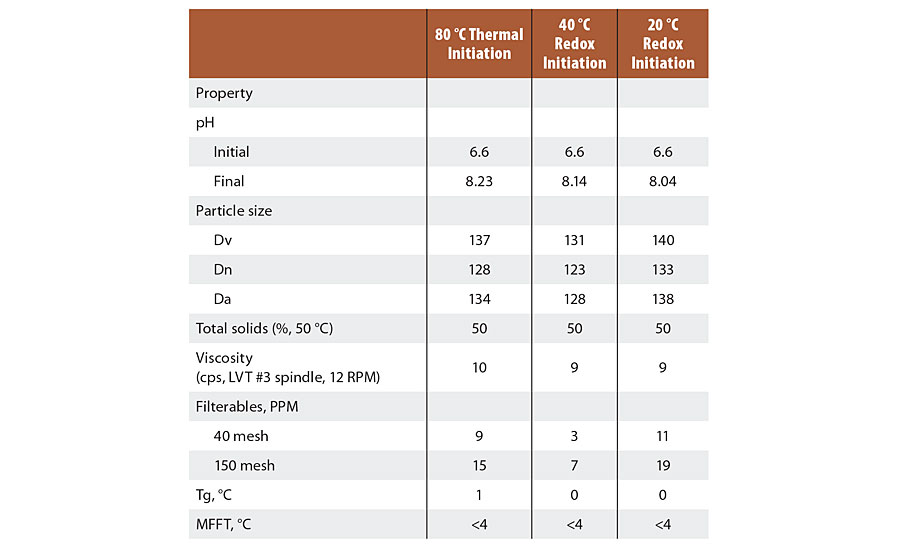
TABLE 6 » Latex properties for Series 2 emulsions.
Molecular Weight Analysis
HPLC conditions for molecular weight analysis were:
- Agilent 1100 system with RI detector.
- Column PLGel mixed D 7.5x300 mm + PLGel mixed C guard 7.5x50 mm.
- Column temperature: 60 °C.
- Mobile phase: THF.
- Flow Rate: 1 mL/min, isocratic.
- 40 µL injection.
The reactions produced higher MW as a function of decreasing temperature of reaction as expected from theory (Peak 1). The Mw increased by over 50% when the temperature was reduced from 80 °C to 20 °C. This is expected to produce improved properties such as scrub resistance. Second peak (Peak 2) is a combination of oligomers and surfactant, and remains constant for the three reaction temperatures. Molecular weight observations are in Table 7.
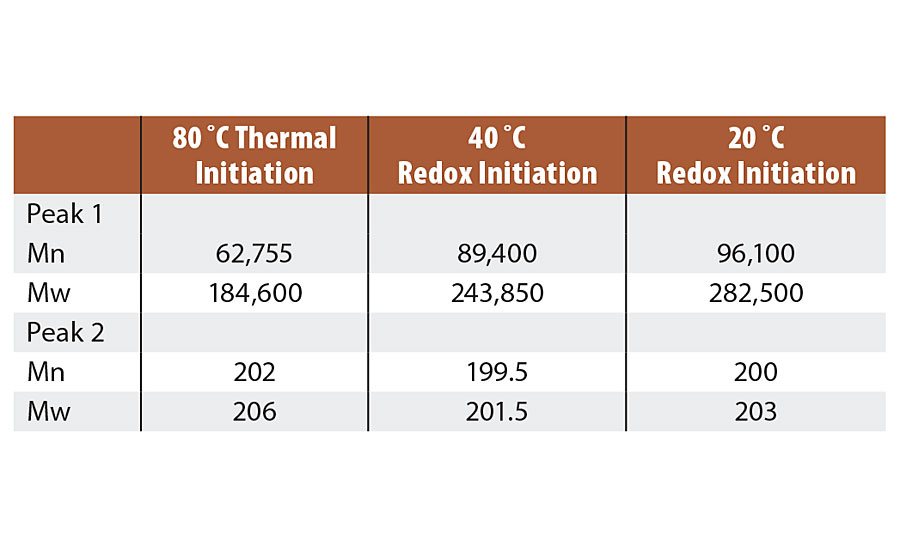
TABLE 7 » Molecular weight of Series 2 emulsions.
Gel Content Analysis
Dried latex specimens were weighed, placed in a 150 mesh stainless steel pouch and then immersed in extracting solvent (THF) for 4 days. After extraction, the specimens were removed, dried and reweighed, and the gel content was calculated. The gel contents were similar between the three reactions. Gel content observed can be found in Table 8.
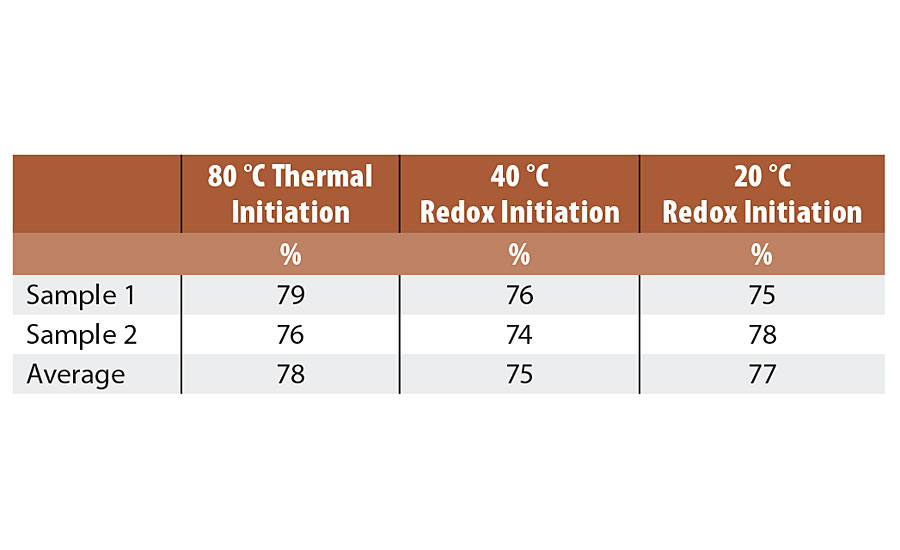
TABLE 8 » Gel content of Series 2 emulsions.
Scrub Data Comparison of Latexes
Resins produced in the 50%-solids cases were formulated into paint. Scrub data for each were compared, using ASTM D 2486 protocol. Results can be seen in Figure 9, revealing a much higher scrub resistance for the latexes produced via low-temperature redox initiation.
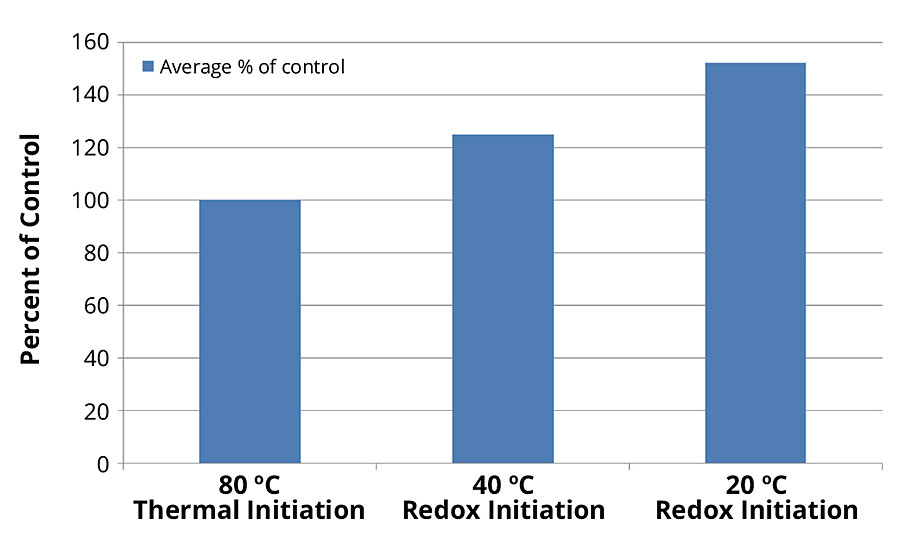
FIGURE 9 » ASTM D 2486 scrub resistance comparison.
Conclusions
There was good process control for both the thermal system reacted at 80 °C and the redox system started at 40 °C. There was no monomer pooling or temperature variation other than a slow rise in temperature from 40 °C to 60 °C. When starting at 20 °C, additional reducer should be introduced in the early stages to prevent monomer pooling.
Similar latex polymer properties were obtained using the redox during the monomer feed starting at 40 °C and 20 °C. The Mw of the latex polymers increased with lower starting reaction temperatures. This resulted in more-durable latex paints. Scrub resistance for the paint formulated with low-temperature redox-initiated latexes was higher than thermal-initiated latex, an increase of 52% for the Series 2 case run at 20 °C. This is due to the increase in molecular weight.
Expected Areas of Value Creation
- Elimination of the heating step of initial reactor charge, reducing energy consumption and time.
- Adiabatic temperature rise during reaction, allowing for faster cycle time since less cooling is required.
- A reduction in reactor fouling is anticipated since the reactor is always in cooling mode. This should reduce “baked on” latex on the sides of the reactor, increasing time between cleanings and shortening time needed to clean reactor.
- Value of yet-unknown properties of latex polymer, although higher Mw usually results in improved toughness and abrasion resistance.
For more information:






Report Abusive Comment