Background on Protective Coatings for Steel
The use of coatings to prevent the corrosion of steel is one of the oldest and most studied areas of industrial science. Committee A05 on Metallic-Coated Iron and Steel Product considers its primary goal to promote the use of metallic and nonmetallic coatings to prevent corrosion. The committee celebrated its centennial anniversary in 2006.1
The ASTM centennial article includes a sidebar on how metallic coatings protect steel from corrosion. 2 There are two mechanisms:
- Barrier Protection – A mechanism to keep water and/or oxygen away from steel;
- Galvanic Protection – An electrochemical force, usually from zinc, preventing steel from corroding by oxidizing in preference to adjacent exposed steel.
Other articles are available on the subject.3, 4 This article will be primarily focused on traditional barrier protection and how breakthroughs in formulation and manufacturing techniques have led to advances in edge corrosion protection.
Barrier protection is not limited to just metallic coatings. Virtually any material can be a protective coating. Consider the important roll biofilms play in the protection of metallic piping in drinking water systems.5 Paints are barrier coatings that add aesthetic appeal. There is a variety of barrier inorganic coatings,6 often referred to as passivations or conversion coatings. These coatings are covalently bonded metal oxides or salts that are more corrosion resistant than the base metal.
With traditional barrier coatings, such as paint, as the number of layers and dry film thickness (DFT) of these coatings increases, the cost increases with it. At this intersection, there is usually a trade-off between the degree of corrosion protection and the cost.
Due to limitations such as this, it is important to be able to protect steel from corrosion with an economical and easy-to-apply coating – passivations and conversion coatings have been the answer in the marketplace. For decades, the optimal combination of ease of application and low cost has been answered by the use of Chromates as the active ingredients in these products.7 Hexavalent chromates Cr(VI) are highly toxic and carcinogenic but are only slowly being replaced due to the poor performance of Trivalent Cr(III) passivations in the marketplace, as compared to the high effectiveness of Cr(VI) passivations.
One of the highly desirable properties of chromates is the ability to repassivate adjacent metal if the barrier coating is damaged. Chromate is sparingly soluble and under corrosive conditions will migrate to nearby metal and repassivate that surface.7 The active chemical in the passivation is Cr(III) oxide or salt.
The following is important in the understanding of the mechanism of the healing process.
- The corrosive media includes water. Chromates have water solubility.
- Once the chromate is in solution, it diffuses and is exposed to metal oxides in electrical contact to the base metal.
- Hexavalent chromate Cr(VI) reacts with the metal oxide/metal by reducing to Cr(III).
- Cr(III) salt deposits onto the metal surface. This is the primary active chemical, not only in chromate passivation but also in stainless steel.
- Cr(III) improves the metal’s resistance to corrosive attack.
Over the years various forms of chromates have been developed to optimize the corrosion protection of chromates. Four of the main improvements have been:
- To use partially reduced Chromates. Some amount of Cr(III) improves the performance of the hexavalent chromate Cr(VI) coating.
- To reduce the solubility of the chromate by depositing it as a metal salt. Typical metal cations are Cr(III), Zn(II), and Sr(II).
- To add a supplemental inhibitor, usually phosphate.
- To imbed the hexavalent chromate Cr(VI) in a material that can act as an additional barrier coating.
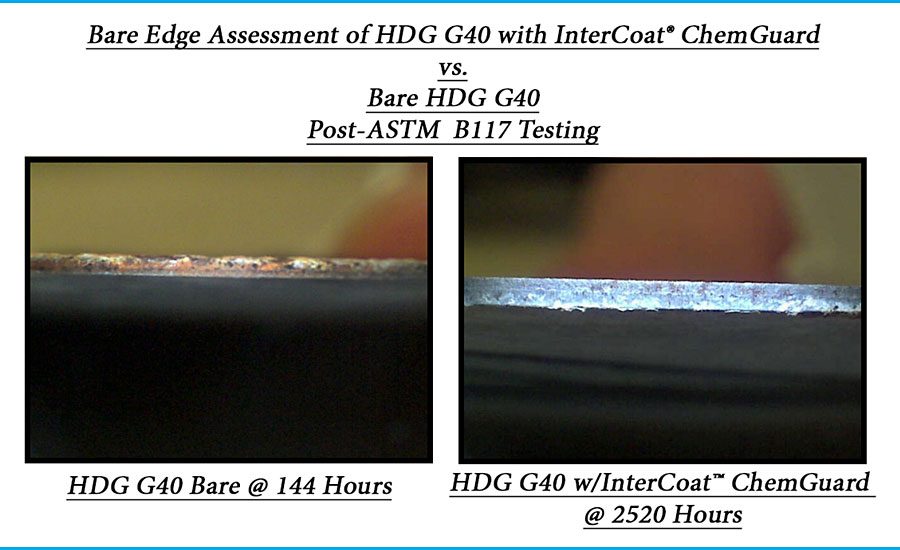
Eco-Green Coatings has found a way to further enhance these four improvements by developing a next-generation product that utilizes only RoHS-compliant trivalent chrome Cr(III) and goes beyond the technology used in traditional barrier coatings, leading to a dramatic improvement in its healing properties. Before explaining in detail, note the shiny steel edge after 2500 hours in NSS in the figure. The extent of the corrosion protection on the edge is surprising, but the answer that follows is based on solid chemical theory. It demonstrates that viewed properly, dramatically enhanced edge protection is entirely expected once the manufacturing limitations were addressed.
Different from traditional steel barrier coatings, InterCoat ChemGuard reacts in a dual-phase manner. At the surface of the substrate, ChemGuard incorporates the metallic zinc into the coating and utilizes it to form a covalent bond. In the upper layers of the coating, additional unreacted components are held within the dry film, providing a reservoir for the coating to “self-heal” with once it has been damaged. This reservoir allows the coating to withstand cold-reduction and to provide corrosion protection to areas damaged by abrasion or cut-edges. The combination of these characteristics together defines what Eco-Green Coatings refers to as “InterReactive® Technology”. In the Table, a cross-section diagram reveals the multiple layers within the Intercoat ChemGuard product.
Characteristics of InterCoat ChemGuard
- Chemguard 300 Series uses 100% reduced Cr(III). When deposited properly, this means that the coating and applied chemical is safer for workers and is less harmful to the environment. The key here is that there is no possibility of the more soluble chromate ions being incorporated into the coating. The rest of the developments are to ensure that it is deposited properly.
- To provide optimal corrosion protection, less-soluble metal ions were chosen. In this case, the more-soluble divalent metal ions have been removed. It makes sense that to get the optimal corrosion protection from a barrier coating the least soluble material needs to be deposited. Chromic phosphate and chromic oxides are far less soluble than the zinc phosphates used in pretreatments or the strontium chromates used in paint pigments.
- Phosphates were chosen to improve the solubility of the Cr(III) – increasing impermeability. By “improve” it is meant that the chemistry is set so that the proportion of chromic ions and phosphate ions are optimal for depositing a highly impervious coating on adjacent areas.
- Inorganic components in deliberate quantities are used to keep the coating and its properties structurally sound during extended corrosion and secondary processing. The chromic and phosphate ions are embedded in an inorganic matrix; much like the old Dacromet passivation9. This matrix holds the coating in place even during corrosive exposure. As slight amounts of Cr(III) and phosphate is released from the coating, the structural integrity and barrier properties of the coating remain intact. This keeps the amount of exposed steel from growing and overwhelming the ability of the coating to passivate adjacent area. This maintaining of an intact coating is the same reason painted Galvalume has less paint loss than HDG with 2-4 times the amount of zinc.
- Application is economical. InterCoat ChemGuard can be applied as low as 0.1 mils per side on HDG G-30 while maintaining superior corrosion performance when compared to HDG G-235 with a hexavalent chrome Cr(VI) treatment. InterCoat ChemGuard corrosion performance directly correlates to dry-film thickness, allowing more demanding applications to use higher coating weights.
- Innovative manufacturing allows conflicting chemistries to exist as one. The preparation of the ingredients for application is what allows this unique chemistry to be applied as a homogenous coating. Chemists recognize this combination of ingredients is exceptionally difficult to maintain in a stable solution. Overcoming this hurdle is the final step in applying a coating to dramatically improve the edge protection properties. Typically, chemists “know better” than to approach this set of chemicals because the solution would not be sufficiently stable for application.
- Unique formulation allows for InterCoat ChemGuard to be modified for unique applications. Welding, cold-reduction and roll-forming/stamping are a few examples of secondary-processing where Eco-Green Coatings can modify their products’ chemistries to better fit the end-use of their customer. Additions like these aid the coating to survive through manufacturing wear-and-tear, resulting in a structurally sound coating that provides general and edge corrosion on finished goods.
The use of coatings to protect steel from corrosion has been and will continue to be a highly important area of industrial science. Barrier coatings and the advancements that stem from them will also continue to be a topic of importance. Barrier coatings, passivations and conversion coatings all offer an array of positive characteristics balanced by faults, such as application limitations, hazardous chemical composition, long-term corrosion failure, and edge/perforation corrosion failure. It is by overcoming hurdles in formulation and manufacturing techniques, that these faults in coating technology are starting to be corrected. These new advances can be observed in products such as Eco-Green Coatings’ InterCoat ChemGuard. When applied as low as 0.05 mil, certified testing results according to ASTM B117 has shown that the 100% Cr(III) InterCoat ChemGuard product can extend the corrosion protection of HDG G40 by more than 10x – while simultaneously showing less scribe and edge corrosion than the leading Cr(VI) treatments in the marketplace.
References
- http://www.astm.org/SNEWS/APRIL_2006/dallyn_apr06.html, Committee A05 on Metallic-Coated Iron and Steel Products, 100 Years of Fighting Corrosion, Gary W. Dallin and Richard F. Lynch
- http://www.astm.org/SNEWS/APRIL_2006/dallynside_apr06.html, How Metallic Coatings Protect Steel, Sidebar to ref 1.
- http://www.ebah.com.br/content/ABAAAfEc4AI/biofilms-strategies-for-metal-corrosion-inhibition-employing, Biofilms: strategies for metal corrosion inhibition employing microorganisms, Rongjun Zuo
- http://ecca.selfware.be/repository/Annina/Basic%20of%20corrosion%20021211.pdf, The Basics of Corrosion, European Coil Coating Association
- http://www.eclectablog.com/2015/10/interview-flint-mayor-dayne-walling-talks-about-flints-water-crisis-emergency-managers-and-the-state-government.html, UPDATED: Flint Mayor Dayne Walling talks about Flint’s water crisis, Emergency Managers, and the State government
- http://www.iso.org/iso/home/store/catalogue_tc/catalogue_tc_browse.htm?commid=51400, ISO/TC 107/SC 8 - Chemical conversion coatings
- https://www.coilcoating.org/download.php/education/toolkits/tool-kit-10, Passivated Steel as a Feedstock to the Coil Coating Process, Tool Kit 10 NCCA
- http://www.pfonline.com/articles/replacing-hexavalent-chromium-in-passivations-on-zinc-plated-parts, Replacing Hexavalent Chromium in Passivations on Zinc Plated Parts, Products Finishing, Craig V. Bishop, Paul C. Wynn
- http://michiganmetalcoatings.com/yahoo_site_admin/assets/docs/Dacromet.16981810.pdf, Darcomet Coating World Class Corrosion Protection, Sales Brochure, Metal Coatings International Inc., Chardon OH. ]
By Eco-Green Coatings






Report Abusive Comment