Dibenzoate glycol esters have been used extensively as plasticizers and coalescent film-forming aids for many years. The advantages of dibenzoates have been discussed previously, but they include: low vapor pressure (in the range of 10-6-10-8 mmHg) resulting in low VOC content, appropriate solubility parameters for applications with polar polymers such as PVC and acrylates, biologically biodegradable, and environmentally safe for food contact applications in adhesives and coatings.1 Previous research presented at the Waterborne Symposium has shown the usefulness of these molecules as film-forming aids for architectural coatings in both interior and exterior applications.2,3 Dibenzoate glycol esters are low-VOC coalescent aids, and their performance advantages in architectural coatings include increased volume solids, gloss and scrub resistance.
This research seeks to expand upon hardness increases found in previous coatings research for direct-to-metal applications and use that concept as a formulary tool for architectural coatings.4 In this previous work dibenzoates and dipropylene glycol n-butyl ether (DPnB) were blended in 1:1 ratios, and the resulting hardness development in a styrene acrylic binder exceeded that of formulations containing 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (TMPDMIB) while containing lower levels of VOC.4 This result was interesting, and warranted further exploration of this performance aspect in architectural coatings.
Hardness development in waterborne coatings has been taught to be critical to block resistance and dirt pickup. The overuse of coalescing aids in a formulation can exacerbate dirt pickup through excessive tackiness of the surface, and the formulation chemist would be wise to optimize use levels. Hardness can be a desirable attribute for several reasons, and this research focuses on evaluating hardness development using various ratios of DPnB with dibenzoates. DPnB is 100% VOC content as evaluated by EPA Method 24. DPnB is a hydrophobic ether with partial solubility in water (4.5 wt%), but its partition coefficient allows DPnB to partition well to the polymer phase to aid in film formation. Using varying ratios of DPnB to dibenzoate, this research will focus on properties resulting from two different binder formulations.
The first resin binder was a higher-Tg styrene acrylic (44 °C) suitable for exterior/interior high-gloss enamel architectural applications. The second binder was a mid-Tg vinyl acrylic (19 °C) with high scrub resistance properties suitable for exterior/interior architectural applications. Both of these binders were formulated as a semigloss coating with a pigment volume concentration of 25%. Hardness development was measured using Koenig for the harder styrene acrylic and Persoz for the softer vinyl acrylic. In addition to hardness development, gloss, block resistance and scrub resistance were also evaluated. By formulating with DPnB in combination with low-VOC dibenzoate esters, some performance properties of coatings can be increased while keeping VOC content at lower levels in comparison to conventional coalescent formulations containing TMPDMIB.
Test Methods
pH
ASTM E70 – The pH of the coatings were measured using a Beckman 310 pH meter with general-purpose electrode. The coatings were pH adjusted to within 8.5 to 9.5 pH using ammonium hydroxide (28%).
Stormer Viscosity
ASTM D562 – Initial Stormer viscosity was measured using a Brookfield KU-2 viscometer with paddle geometry. Rheology modifier was added to adjust initial viscosity to within the range of 90-105 KU.
MFFT
ASTM D2354 – Minimum film formation temperature (MFFT) was evaluated using a Gardco MFFT Bar 90 instrument. Polymer latex emulsions blended with nonionic surfactant and coalescent were drawn down using a MFFT draw down applicator, and film formation evaluated after 1 hr. The temperature gradient setting on the instrument was -5 °C to 13 °C. The film formation temperature was evaluated visually and the temperature measured using a separate temperature probe.
Scrubbability
ASTM D2486 – Coatings were applied using a 7-mil Dow applicator bar to a Leneta P121-10N chart and dried at 23 °C at 50% RH for 7 days. The scrubbability was measured using a Gardco D10 Washability and Weartester. A 10-mil shim was employed with abrasive media (SC-2). Initial failure was recorded, and complete failure defined as a continuous thin line across the shim.
Block Resistance
ASTM D4946 – Coatings were applied using a 3-mil Bird film applicator to a Leneta form WB chart and dried in an environmentally controlled room at 23 °C and 50% relative humidity for 7 days. Samples were constructed from 1.5-inch squares and oriented coating surface to coating surface with a 1 kg weight placed upon a number 8 stopper at ambient temperature or 120 °F for 30 min. The samples were then allowed to equilibrate at room temperature for 30 min and were then evaluated through “blind” testing to remove bias.
Gloss
ASTM D2243 – Coatings were applied using a 3-mil Bird film applicator to a Leneta form WB chart and dried in an environmentally controlled room at 23 °C and 50% relative humidity for 7 days. Gloss measurements were conducted in triplicate using a Gardco micro-Tri-gloss meter model 4446.
Hardness Development
ASTM D4366 – Coatings were applied using a 3-mil Bird film applicator to aluminum A36 Q-PANEL and dried in an environmentally controlled room at 23 °C and 50% relative humidity. Hardness was measured using a Gardco Koenig and Persoz Hardness Rocker with the respective pendulums for each test. Hardness values were reported as the average of three measurements.
Results and Discussion
Ternary blends of binder, surfactant and coalescent were prepared to determine appropriate coalescent loadings and to assess partitioning. This is often performed in a new coalescent platform to ascertain the loading required in the finished paint formulation to achieve a MFFT of less than 40 °F (4 °C). Background work, not reported here, established the appropriate coalescent and nonionic surfactant loading for each polymer emulsion according to MFFT performance. An 8 wt% coalescent and 1 wt% nonionic surfactant with respect to the binder was found to be optimal for the high-Tg styrene acrylic emulsion. A 2 wt% coalescent and 0.25 wt% nonionic surfactant with respect to the binder was found to be optimal for the mid-Tg vinyl acrylic emulsion. These ratios of coalescent loading to binder were then used in the full coating formulations. The coating formulations are detailed in Table 1 and Table 2. The dibenzoate coalescent evaluated in this study was 850S, a blend of diethylene glycol dibenzoate and dipropylene glycol dibenzoate. The four coalescent blends in Figure 1 outlines the coalescent blending design space for MFFT performance for the coalescent blends used in this study. This was useful in that MFFT performance of varying coalescent ratios should lie within this performance window, making measurement of every blend ratio unnecessary.
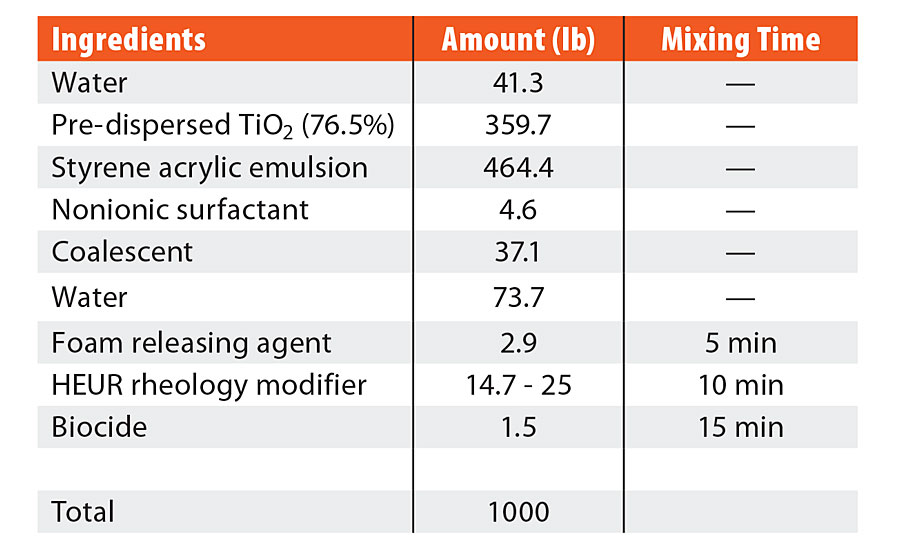
TABLE 1 » Formulation ingredients for high-Tg styrene acrylic.
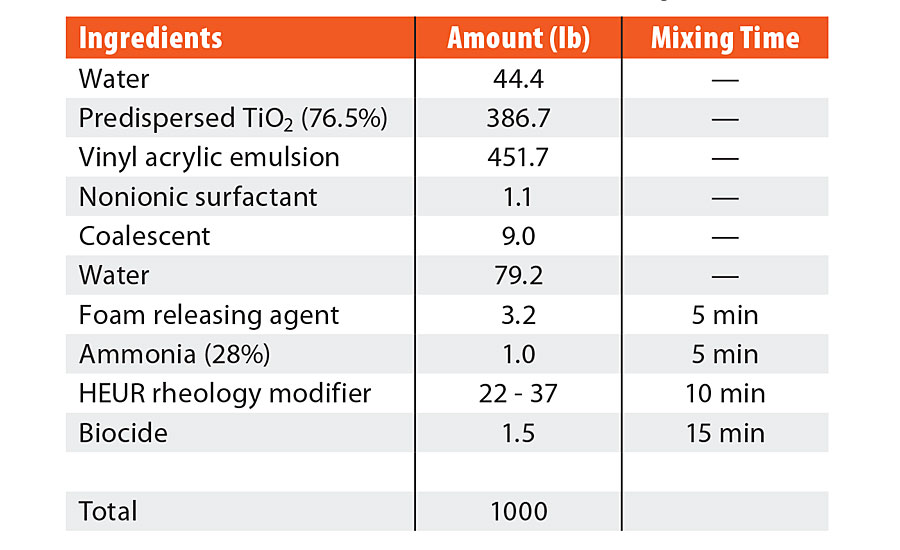
TABLE 2 » Formulation ingredients for mid-Tg vinyl acrylic.
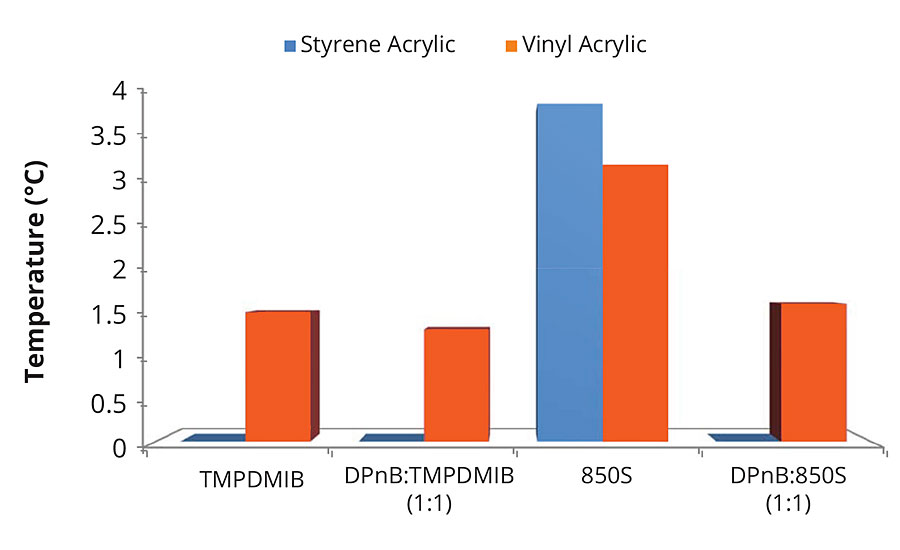
FIGURE 1 » Minimum film formation temperatures for high-Tg styrene acrylic and mid-Tg vinyl acrylic ternary mixtures with nonionic surfactant. Coalescent loading is 8 wt% in addition to 1 wt% nonionic surfactant with respect to the styrene acrylic binder, or coalescent loading of 2 wt% in addition to 0.25 wt% nonionic surfactant with respect to the vinyl acrylic binder.
The coating formulations were created with the ingredients listed in both Table 1 and Table 2. The ingredients were added sequentially, as listed in the table, with a dispersion blade mixer head at an appropriate speed to maintain an adequate vortex (approximately 700 rpm). Six coalescent platforms were evaluated for each formulation: TMPDMIB, DPnB:TMPDMIB (1:1), 850S (blend of diethylene glycol dibenzoate and dipropylene glycol dibenzoate), DPnB:850S (1:5), DPnB:850S (1:2), and DPnB:850S (1:1). Dipropylene glycol n-butyl ether was considered to be an equal contributor to the total coalescent in the formulation, thus the sum of the varying ratios of coalescent equaled the coalescent weight listed in Table 1 and Table 2. In each formulation, DPnB was added prior to the corresponding coalescent. After addition of the foam-releasing agent, mixing times were used to promote complete dispersion and equilibrium before the next addition, as listed in Table 1 and Table 2. The pH was adjusted with ammonium hydroxide to be within 8.5 to 9.5 and was found only to be necessary for the vinyl acrylic formulation. The rheology modifier was added to adjust the Stormer viscosity to within range of 90 to 105 KU. Increasing amounts of rheology modifier were needed as additional DPnB was added to the formulation, with the range listed in Table 1 and Table 2. The rheology modifier was a hydrophobically modified ethoxylated urethane resin (HEUR) type and DPnB may have caused inhibition of the hydrophobically associated network since DPnB is partially water soluble (4.5 wt%). After formulation, the coatings were allowed to equilibrate for three days prior to drawdowns and physical testing.
The VOC content of each formulation was calculated, assuming that TMPDMIB and DPnB were 100% VOC according to EPA Method 24 as measured previously.5 The VOC content for 850S has been published previously (2.2 wt%)6 and was used to estimate VOC contribution. The only other known VOC contributor in the formulations was ammonium hydroxide used to pH adjust the vinyl acrylic coating. The calculated VOC content of each formulation is listed in Figure 2.
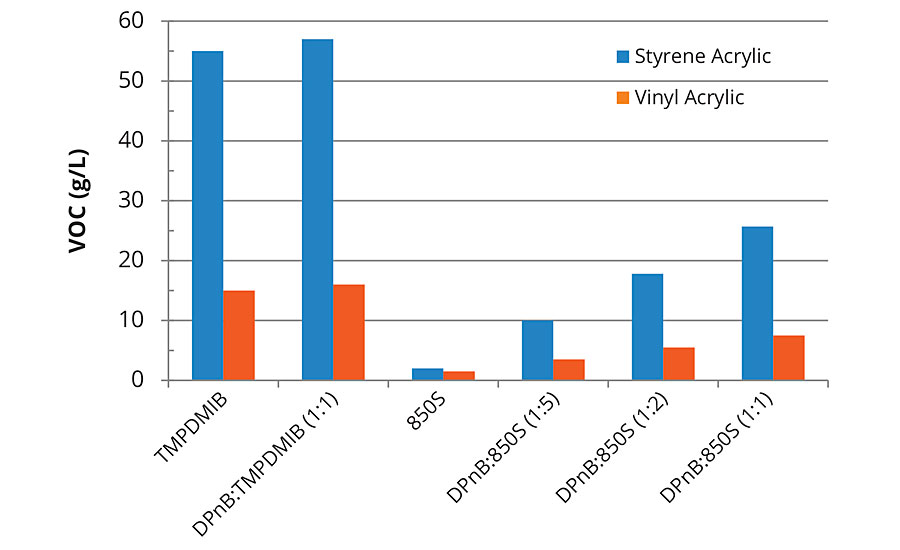
FIGURE 2 » Calculated VOC content including water for high-Tg styrene acrylic and mid-Tg vinyl acrylic formulations. Coalescent loading is 8 wt% of the styrene acrylic binder and 2 wt% of the vinyl acrylic binder.
Figure 3 shows the Koenig hardness increase in the high-Tg styrene acrylic formulation over time. This data clearly illustrates increasing hardness as a function of increasing DPnB content. Hardness increases are observed even in the DPnB:TMPDMIB (1:1) formulation. Hardness development equals or exceeds TMPDMIB performance in formulations of DPnB:850S (1:2) and DPnB:850S (1:1) even though VOC content was at lower levels in these formulations.
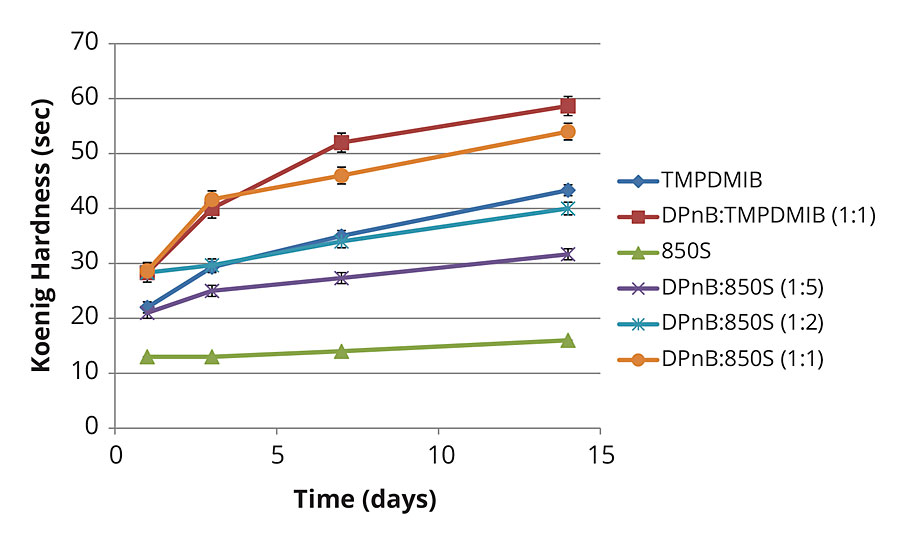
FIGURE 3 » Hardness development of high-Tg styrene acrylic coating measured with Koenig pendulum using method ASTM D4366.
Figure 4 illustrates Persoz hardness development in the softer vinyl acrylic. In this softer vinyl acrylic binder, equal Persoz hardness is achieved between 850S and TMPDMIB. The trend parallels that in Figure 3, where increasing DPnB to 850S ratios results in increasing hardness of the coating at lower VOC content compared to TMPDMIB.
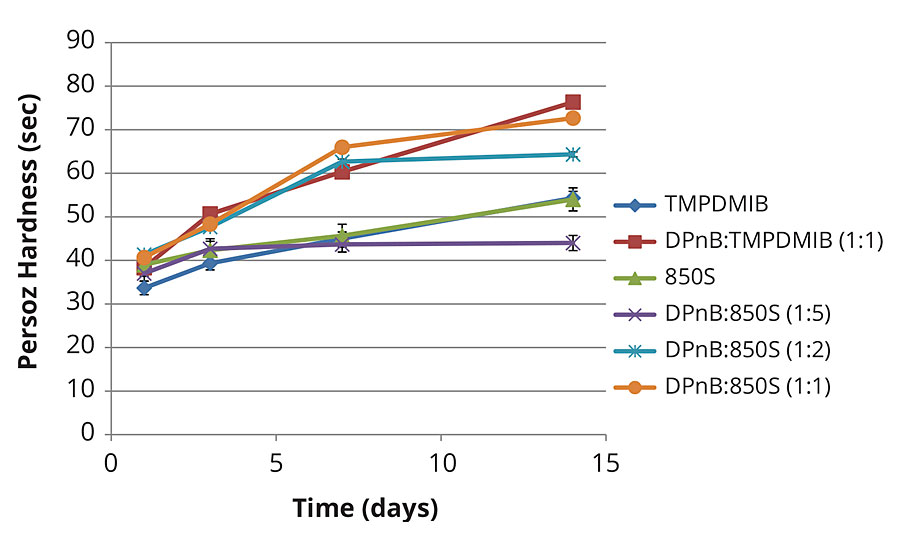
FIGURE 4 » Hardness development of mid-Tg vinyl acrylic coating measured with Persoz pendulum using method ASTM D4366.
The trends observed in Figure 3 regarding hardness development of the styrene acrylic are reflected in the block resistance data in Figure 5 and Figure 6 for 1-day and 7-day measurements. Block resistance was determined through blind evaluation to remove bias. From the data in Figures 5 and 6, block resistance increases with DPnB content, which also correlates to increasing hardness seen in Figure 3. Measurements conducted at ambient temperature and 120 °F both trend to increasing hardness at higher DPnB content. Figure 6 reports block resistance after 7 days at 23 °C and 50% relative humidity. High block resistance ratings were observed in the DPnB:850S ratios (1:2) and (1:1), which have one-third to one-half the VOC content of TMPDMIB.
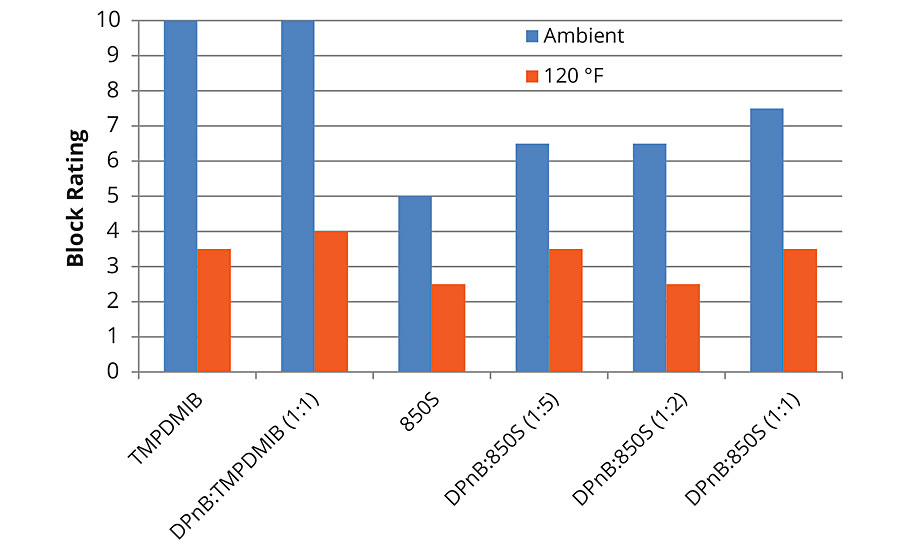
FIGURE 5 » Average block resistance after 1 day for high-Tg styrene acrylic measured using ASTM D4946 in a blind evaluation to remove bias.
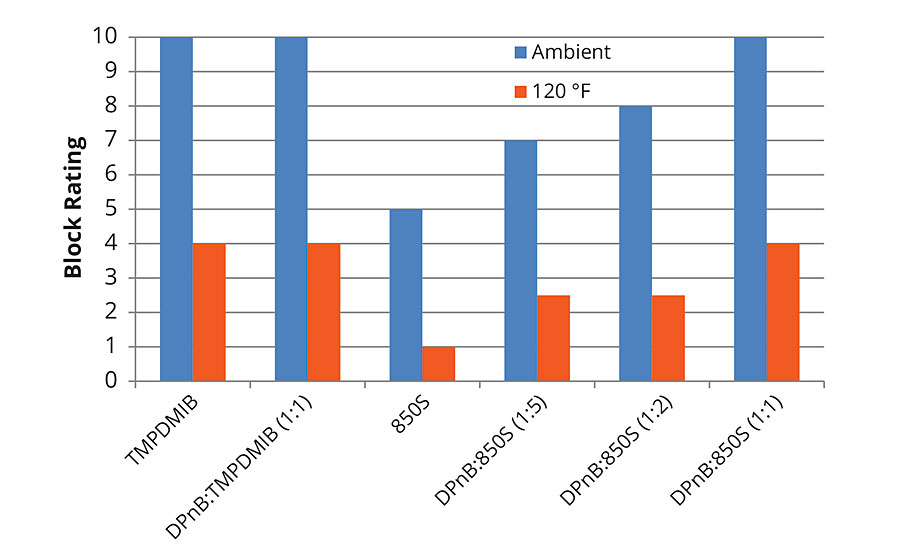
FIGURE 6 » Average block resistance after 7 days for high-Tg styrene acrylic measured using ASTM D4946 in a blind evaluation to remove bias.
Block resistance of the softer vinyl acrylic semigloss was poor in each of the formulations tested, with the dibenzoate blend 850S being on average better than the other examples as shown in Figures 7 and 8 for 1-day and 7-day measurements. For 7-day evaluations, the DPnB:TMPDMIB was equal to 850S taking into account ambient and 120 °F tests. In the softer vinyl acrylic, there was no correlation between hardness development and block resistance as evidenced in the styrene acrylic.
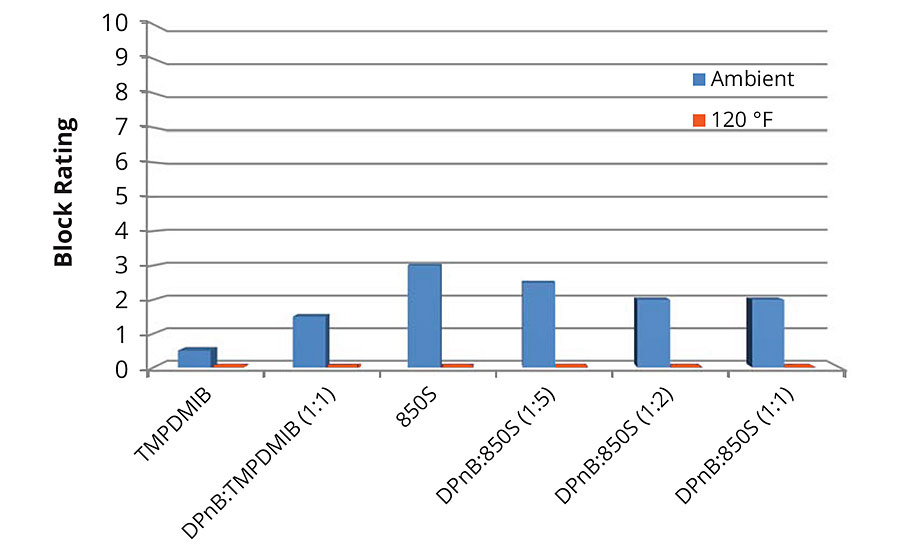
FIGURE 7 » » Average block resistance after 1 day for mid-Tg vinyl acrylic measured using ASTM D4946 in a blind evaluation to remove bias. Data collected at 120 °F resulted in block ratings of zero.
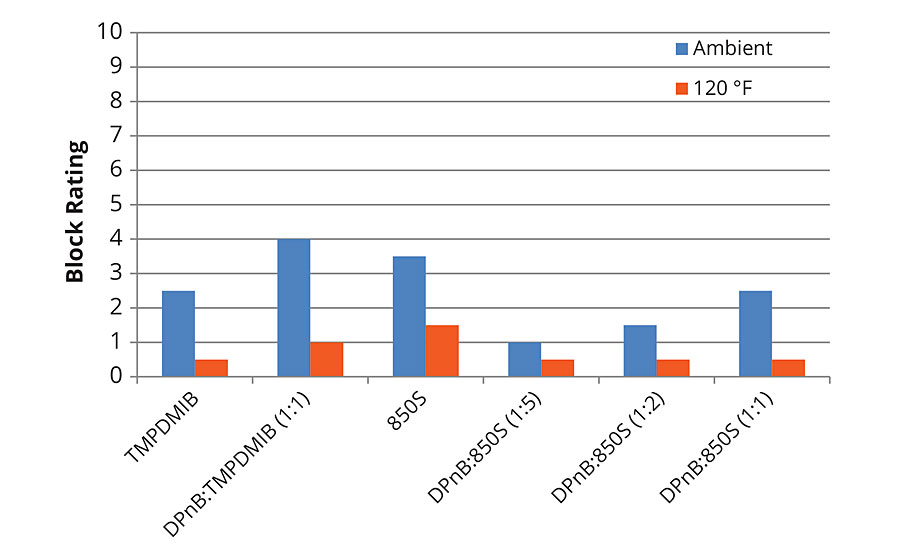
FIGURE 8 » Average block resistance after 7 days for mid-Tg vinyl acrylic measured using ASTM D4946 in a blind evaluation to remove bias.
Dibenzoate glycol esters offer advantages in producing higher gloss levels, allowing higher content of pigment extenders in formulations. Figure 9 illustrates this concept in the gloss values of the styrene acrylic being considerably higher than TMPDMIB. As DPnB is increased, the gloss values decrease, but still maintain higher values than TMPDMIB and at lower VOC content. This same trend is mirrored in the vinyl acrylic, with DPnB:850S (1:5) being slightly higher than 850S. As the DPnB concentration increases, the gloss values of the coatings decrease slightly, but still remain higher than TMPDMIB.
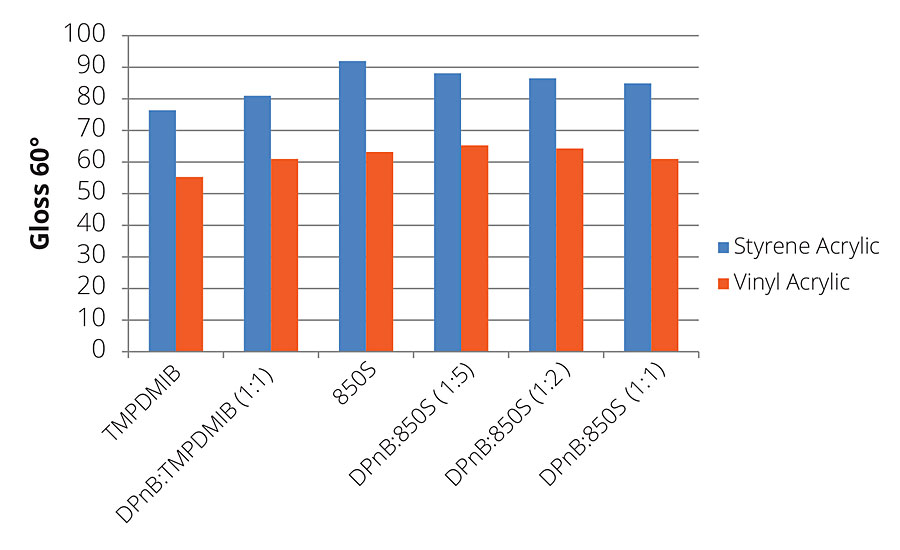
FIGURE 9 » 7-day gloss for high-Tg styrene acrylic and mid-Tg vinyl acrylic formulations.
In addition to gloss, dibenzoates usually offer performance advantages for coatings in scrub resistance in comparison to TMPDMIB. However, for the high-Tg styrene acrylic binder used in this research, 850S resulted in slightly lower scrub resistance using ASTM D2486 with abrasive media, as shown in Figure 10. While hardness development has been shown to increase with DPnB:850S ratios, scrub resistance was reduced in the styrene acrylic coating at higher ratios. The styrene acrylic performance was contrasted by the dramatic scrub resistance increase observed in the vinyl acrylic coatings shown in Figure 11. The increase in performance due to the dibenzoate ester blend was significant compared to TMPDMIB and DPnB:TMPDMIB (1:1) with this binder. The formulation that produced the highest scrub resistance was DPnB:850S (1:2), however, a replicate experiment was unable to be obtained.
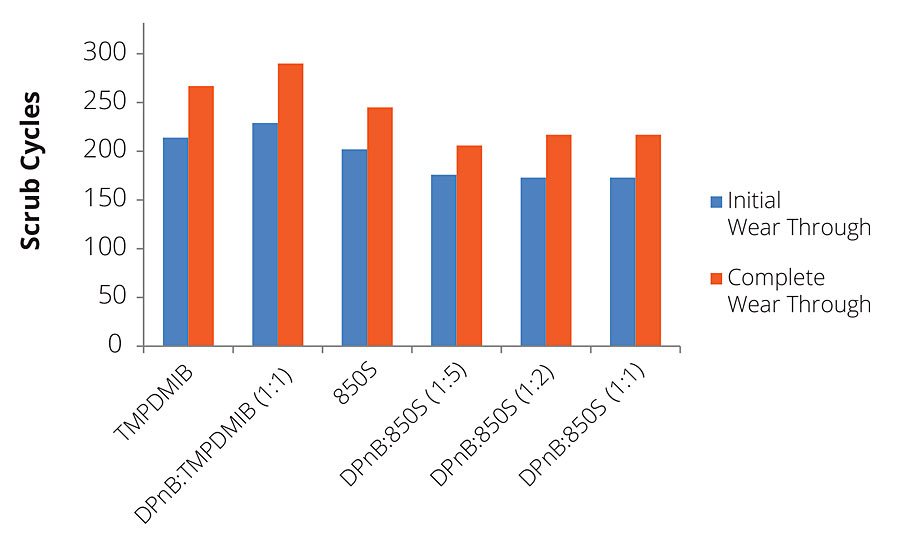
FIGURE 10 » Scrub resistance for high-Tg styrene acrylic measured using ASTM D2486 with abrasive media and shim.
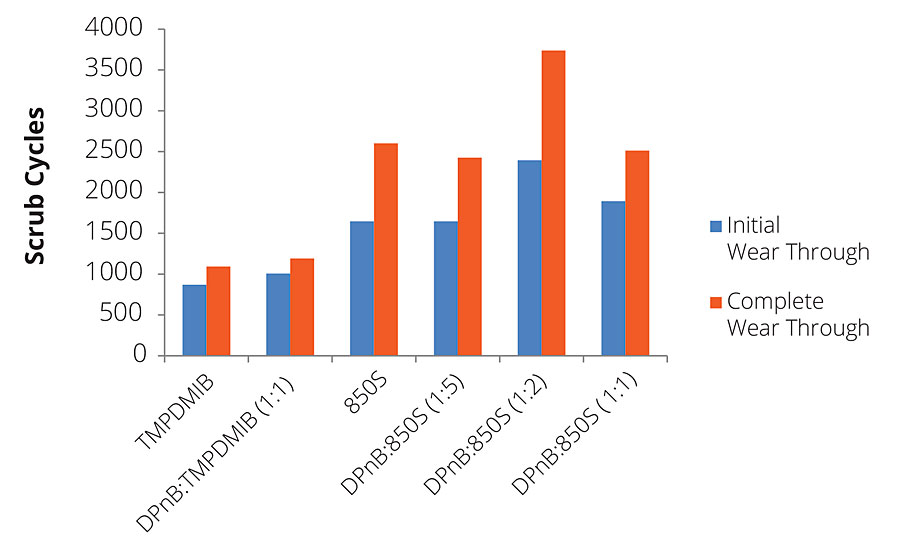
FIGURE 11 » Average scrub resistance for mid-Tg vinyl acrylic measured using ASTM D2486 with abrasive media and shim. A replicate experiment was unable to be obtained for DPnB:850S (1:2).
Conclusions
Architectural low-VOC coatings can be formulated with dibenzoate glycol esters to have increased hardness, block resistance, gloss and scrub resistance. The low VOC content of the dibenzoates allow formulators freedom of design to include higher-VOC components in their coatings, achieving various properties that are critical to specific applications. This research focused on only two binders in a semigloss model formulation to illustrate coalescent polymer properties, but the concept presented here would work with different polymers and pigment volume concentrations. Formulating with DPnB and 850S allowed higher hardness development, higher block resistance, maintained higher gloss values and significantly increased scrub resistance in the vinyl acrylic.
References
1 McBride, E.; Arendt, W.D.; Conner, M. Sticking to the Regulations: Advantages of Benzoates in Waterborne Adhesives for Food Contact, Eur. Coat. J. 2014, 6, 28-31.
2 Arendt, W.D.; Conner, M.; McBride, E. Case Studies in Formulating with Low-VOC Coalescents, Proceedings of the 42nd Annual International Waterborne, High-Solids and Powder Coating Symposium, 2015, New Orleans, LA.
3 Arendt, W.D.; McBride, E.L.; and Johnson, G.M. Longer Term Fence Performance of Exterior Architectural Paint, Proceedings of the 43rd Annual International Waterborne, High-Solids and Powder Coating Symposium, 2016, New Orleans, LA.
4 Macy, G.; Arendt, W.D.; Conner, M.; McBride, E. Continuing Development of Low VOC Benzoate Coalescents High PVC Interior Grade, Primer, Direct-to-Metal Coatings, Western Coatings Symposium, Las Vegas, NV, October 25 - 28, 2015.
5 Arendt, W.D.; McBride, E. New Dibenzoate Plasticizer/Coalescent Blends for Low-VOC Coating Formulations, Proceedings of the 38th Annual International Waterborne, High-Solids and Powder Coating Symposium, 2011, New Orleans, LA.
6 Arendt, W.D.; McBride, E.L.; Conner, M.M. Low-VOC Dibenzoate Triblend and Dibenzoate Diblend in Exterior Coatings, Proceedings of the 40th Annual International Waterborne, High-Solids and Powder Coating Symposium, 2013, New Orleans, LA.
For more information, e-mail Stephen.Foster@emeraldmaterials.com. This paper was presented at the 2017 Waterborne Symposium.





Report Abusive Comment