
Environmental considerations have stimulated research on many high-performance pigment surface modifications as well as the applications of polymeric dispersants to improve rheological characteristics of modern high-solids solventborne pigment dispersions. The approach involves adsorption of specific substituted derivatives (anchors) onto the pigment surfaces to maximize interaction with appropriate polymeric dispersants, resulting in significant rheological improvements and concomitant improvement in aesthetic values of resulting coatings.
In colored paints, the pigments make the greatest contribution to the viscosity of the liquid system. Acceptable and stable viscosity is a prerequisite to a stabilized system that will deliver the required aesthetics to the ultimate automotive finish. Any pigment modification that provides system rheological improvements is desirable. Lowered viscosity allows an increase in pigment concentration and, therefore, a reduction of emissions to the atmosphere.
Reduction of solvent levels in coatings manufacture is an attractive, effective and necessary concept. A large reduction in binder viscosity was accomplished many years ago when a significant part of the U.S. automotive industry moved from lacquers to enamels. Lowering the molecular weight of binders and introducing appropriate compounds for subsequent crosslinking made a contribution toward lowering viscosity. In addition, surface modification of pigments and the use of compatible polymeric dispersants have made2 an important contribution toward lowering paint viscosities.
Although new technologies are being developed to reduce or eliminate solvent emissions, such as powder coatings, ultraviolet light or electron beam curing of paints, these technologies show benefits as well as drawbacks, such as the requirement of large capital investments.
Waterborne paints are making inroads in the automotive industry but solventborne counterparts still make up about 65% of total U.S. production. The current study was undertaken in an effort to provide significant improvements in solventborne systems for a series of high-performance pigments to be used primarily but not exclusively in automotive coatings. This article presents an evaluation of polymeric dispersants in conjunction with surface modified pigments.
Dispersion Preparation
Pigment dispersion preparation involves both skill and experience. A solid powder consisting of agglomerated and aggregated pigment particles must be dispersed in a liquid. It is essential to break up pigment agglomerates and aggregates through application of sufficient force by shear and/or impact. This mechanical breakdown of the pigment assemblies produces a dispersion of fundamental, separate single pigment particles in order to develop maximum tinctorial properties such as color strength, opacity or transparency. When dispersion is achieved, it must be stabilized to lead to lower viscosity, enhanced gloss and distinctness of image (DOI) of an ultimate finish.
In the present study, we dealt with dispersions of high-performance pigments of varying particle size in a solventborne system of relatively low dielectric constant. Unless there is a deliberate attempt to stabilize dispersions during their preparation, both the duration and cost of dispersion are likely to rise. In addition, the ultimate dispersion attributes are negatively affected by high surface energies or van der Waals forces that cause particle attractions, usually leading to reformation of agglomerates or pigment particle assemblies of individual primary particles. This is the fundamental cause of undesirable flocculation. This article describes measures taken to stabilize dispersions of four different pigments with and without surface modifications.
Pigments Studied
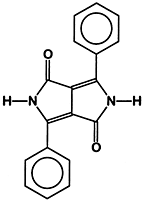
The most recent addition of a major class of new high performance pigments are the 1,4-diketo-3,6-diarylpyrrolo[3,4c]pyrroles (DPPs). The aryl groups may be simply phenyl moieties (see Figure 1) in the parent compound (P.R. 255), or di(p-chlorophenyl) in P.R. 254, or di(biphenyl) in P.R. 264, etc.Substituent groups cause shifts in the color of the pigments as a function of their nature and position on the phenyl rings.
P.R. 254 optimized for opacity is a yellow shade red pigment of excellent saturation and outdoor durability. Since it is designed for solid color stylings, it is a relatively large particle size pigment with a surface area of 13.6 m2/g. Naturally, most pigments have a definite particle size distribution; an opaque pigment like P.R. 254 optimized for particle size would have most particles approximating half the major wavelength of absorption in the visible spectrum of 517 nm or about 0.26 _m in size.
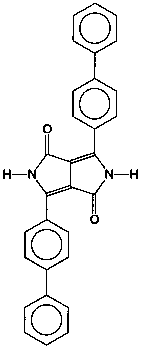
P.R. 264 (see Figure 2) is a blue shade red pigment with a surface area of 29.3 m2/g. It is a very opaque and weatherfast pigment. A smaller particle size transparent version of the same chemical structure with a surface area of 75.0 m2/g was also studied.
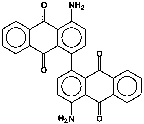
P.R. 177 (see Figure 3), 4,4’-diamino-1,1’-dianthraquinonyl, is a small particle size, transparent blue shade red, saturated pigment. Its surface area was also found to be 75.6 m2/g. All pigments in this study are used predominantly but not exclusively in automotive finishes.

Pigment Surface Modification
Surface modification has been used successfully with other pigment classes.2,3 The diketo-pyrrolopyrrole pigments in the present study have highly ordered crystalline structures that are driven by strong intermolecular hydrogen bonding and stacking forces between adjacent planar molecules.4 A similar situation occurs with the anthraquinoid pigment that shows strong intra- as well as intermolecular hydrogen bonding due to the four hydrogens on the amino functions.5Desirable derivatives (anchors) of the compounds that make up the pigment and retain the ability to hydrogen bond with the pigment surfaces are made available for firm attachment to such surfaces. These specific pigment surface adhering treatments must have the ability to form relatively strong associations with specifically selected polymeric dispersants. We have treated the surface of opaque P.R. 254 and opaque P.R. 264 with an acidic or potentially acidic derivative of DPP, which is readily adsorbed onto pigment surfaces, thus providing pigment particle surfaces with anchor groups. The surface treatment of the pigments is introduced in the pigment manufacturing process and the polymeric dispersants into the pigment dispersion stage.
Polymeric dispersants can be obtained from a variety of sources such as EFKA Additives (EFKA[R]), Avecia (Solsperse[R]), Byk Chemie (Disperbyk[R]), and others, which are designed for non-aqueous systems.
Rheological Studies
Opaque Pigments
In making all dispersions, 20% of specific polymeric dispersants based on pigment weight were included. The weight ratio of dispersant to pigment was not optimized in this study; rather it was kept constant to avoid introducing another variable.
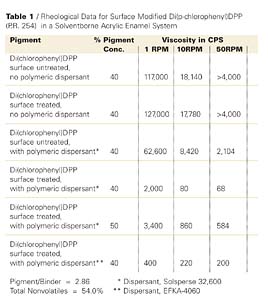
The dispersants are polymers that carry basic moieties such as tertiary amino functions of aromatic or non-aromatic character on a polyurethane chain (EFKA-4060) or attached to a modified polyacrylate polymer (EFKA-4400) in EFKA Additives’ dispersants or a polybasic comb structured polyester in Avecia’s Solsperse 32,600. Following the wetting of the pigment and particle deagglomeration during the dispersion preparation, individual particles carrying anchor groups become exposed and interact with the polymeric dispersants that are usually block copolymers of limited molecular weight. In effect the phenomenon of molecular recognition comes into play. Results of dispersion viscosity measurements at several shear rates are recorded in Table 1 (also see the data of the first 4 entries shown in graphic form in Figure 4).

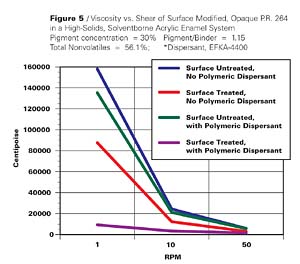
Opaque Pigment Red 264, a structurally related pigment, was similarly treated and yielded the results shown in Figure 5 and Table 2.
The effect in this case is not as pronounced as in the previous example. Nevertheless, it is only when the surface treated di(biphenyl)DPP pigment is dispersed in the presence of an appropriate polymeric dispersant, that we see a significant reduction in viscosity. The reduction is 17 fold at a shear of 1 RPM and 4 fold at 50 RPM. This type of rheological behavior makes the surface treated pigment usable at current emission requirements.

Transparent Pigments
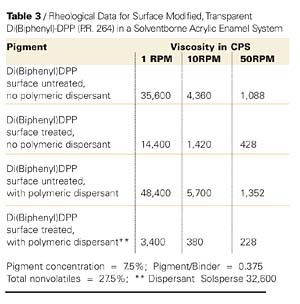
So far, we dealt with opaque pigments; however, this approach also applies to small particle size, transparent counterparts. Thus, a similar effect has been demonstrated with the small particle size, transparent diketo-pyrrolopyrrole pigment (P.R. 264). However, its dispersion had to be carried out at a significantly lower pigment concentration. The data is shown in Figure 6 and Table 3.Again, it is apparent that to obtain the lowest possible viscosity profile, it is necessary to use the surface treated pigment in conjunction with an appropriate polymeric dispersant. It is further apparent from electron microscopy that the transparent surface treated pigment dispersed in the presence of a polymeric dispersant (see Figure 7) is effectively deflocculated. By contrast (see Figure 8), a similar electron micrograph obtained on a dispersion of the same surface untreated pigment and dispersed in the absence of a polymeric dispersant shows large pigment particle assemblies.
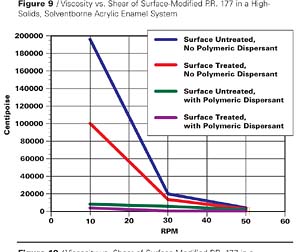
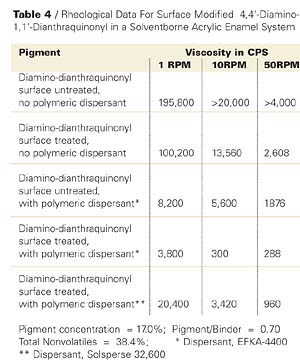
Another small particle size, transparent pigment similarly studied is P.R. 177. In this case, an appropriate acid derivative of the 4,4’-diamino-1,1’-dianthraquinonyl was utilized for application to the pigment surface, and in the dispersion step basic polymeric dispersants were included. The rheological results are shown in Table 4 and in graphic form of the first 4 entries in Figure 9.
The results in this case are similar. Although the inclusion of EFKA-4400 dispersant with the surface untreated pigment shows a significant viscosity reduction, it is only with the surface treated counterpart that we observe another viscosity decrease and obtain a dispersion that shows nearly Newtonian flow. This is more accurately demonstrated in a comparison of viscosities of the latter two cases on a different scale in Figure 10.
In addition, it is noteworthy that surface treated P.R. 177 dispersed without inclusion of a polymeric dispersant produced a two coat (clearcoat superimposed on a colorcoat) masstone (fulltone) with a distinctness of image (DOI) of 45.7, whereas upon inclusion of two different polymeric dispersants, the coatings’ DOIs were 83.3 and 83.0 for the two dispersants shown in Table 4, respectively. The surface untreated counterpart with or without polymeric dispersants showed significantly lower DOIs than those given in the presence of the two polymeric dispersants. A coating showing total specular reflection would produce a DOI of 100. Thus, the lowered viscosity not only permits an increase in pigment concentration and thus a reduction in VOC and HAP, but due to deflocculation of the dispersed pigment, considerably better finish aesthetics.

Stabilization Mechanism
Since organic pigments such as P.R. 254, P.R. 264 or P.R. 177 are reasonably non-polar substances, they tend to adsorb binder resin, which could provide a modicum of steric inhibition to flocculation and consequently lower viscosity. Unfortunately, the attachment between pigment and binder polymer is often reasonably weak;6 usually simple physical adsorption shows a dissociation energy of about 2 kcal/mole, or by hydrogen bonding of totally disparate structures the bond strength is about 7 kcal/mole, leading sometimes to significant disassociation or desorption. This is particularly true for current enamel low molecular weight polymers used in the dispersion stage of automotive finishes. Consequently, a need has arisen to provide a way to increase the bonding force between such pigment particles and paint polymers. The most effective method is utilization of the process of chemisorption employed in the present study. Chemisorption is defined as a process by which molecules, atoms or ions of a gas or liquid are attached to the surface of a solid material. In the present case, the attachment is to solid pigment particles by special polymers in solution.
Particular derivatives of compounds, which make up specific pigments, were synthesized. These derivatives were easily adsorbed onto the pigment surfaces because of their affinity for the substrates, as a consequence of similarity of structures and their ability to hydrogen bond with molecules on the surface of the pigment particles. If the derivative is acidic or potentially acidic in nature the polymeric dispersant is preferably basic in character. If the derivative can be characterized as a base, the polymeric dispersant is preferably acidic in character. The bonding group of the polymeric dispersant is attached to a polymeric chain of considerable solvency. These requirements are imposed if one is searching for very pronounced viscosity lowering effects. When the proper selection is made, the two chemical entities interact in the process of dispersion preparation and form a reasonably strong bond with presumed dissociation energies of about 20 kcal per mole or higher.7

Conclusion
Laboratory experiments have demonstrated significant viscosity reduction effects when several high performance surface treated pigments were dispersed in a solventborne acrylic system in the presence of several different polymeric dispersants. The latter were selected for their ability to undergo chemisorption onto the surfaces of the pigment particles. Significant viscosity reductions were achieved in all cases, the degree of improvement varying with the nature of the pigment. This combination of pigment surface treatment and interactive polymeric dispersant is postulated to inhibit the reagglomeration and subsequent flocculation of individual pigment particles, resulting in lowering of the systems’ viscosities.
Acknowledgement
The authors thank T. Landuydt and S. Jerusik of Ciba Specialty Chemicals for consultation and analytical support, respectively.We also appreciate the assistance of G. Woods from EFKA Additives and R. Slater from Avecia for supplying samples and information concerning the use of dispersants.
Solsperse is a registered trademark of Avecia Limited. Disperbyk is a registered trademark of Byk Chemie GmbH.
For more information on organic pigments contact Ciba Specialty Chemicals Corp., 205 South James St. Newport, DE 19804.
References
1 Concentrates, Chemical and Engineering News, p. 18, Oct. 29 (2001).2 Jaffe, E.E., et al, J. Coatings Technol, 66, No. 832, 47-54 (1994).
3 Vernardakis, T.G., Modern Paint and Coatings, 75, 32-36 (1985).
4 Iqbal, A. et al, J. Coatings Technol, 60, No. 758, 37-45 (1988).
5 Ogawa, K.; Scheel, H.J. Z. Kristallogr. Kristallgeometrie, Kristallphys., Kristallchem., 130 (4-6), 405-419, (1969).
6 Wheland, G.W. Advanced Organic Chemistry, Second Edition, John Wiley and Sons, Inc. pp. 10, 49 (1953).
7 Gladstone, S. Physical Chemistry, second Edition, D. Van Nostrand Co., pp. 1201-1205 (1946).
8 Doroszewski, A.; Lambourne, R, Polymer Science, Part G No. 34, 253-264 (1971); and J. Colloid and Interface Science, 43, No.1, 97-104 (1973).
9 Walbridge, D.J., in book by Barrett, K.E.J., “Dispersion Polymerization in Organic Media”, Chapter 3, pp. 45-114 (1975).
10 Hampton, J.S. Modern Paint and Coatings, 75, 46-54 (1985).
Cowley, A.C.D. J. Oil and Colour Chemists Assoc, 70, (8) 207-213 (1987).



Report Abusive Comment