On the other hand, new high-molecular-weight, epoxy-based waterborne cationic primers show excellent barrier properties that can effectively prevent topcoats from staining and retarded drying.
The amount and the composition of tannin in wood are not uniform; it depends on the species of tree and on the region where it has grown. The main component is penta-meta-digalloylglucoside, a derivative of gallic acid (3,4,5-trihydroxybenzoic acid). Colorless solutions of gallic acid, especially of the alkali metal salts, turn brown in contact with air. With iron, an intensive, colored precipitate is formed. When exposed to moisture, tannins migrate through the wood and waterborne coatings, depositing on the coating surface and creating yellow or brown discolorations.
In the past, several attempts have been made to overcome the bleeding problem of waterborne coatings on wood. One approach was the use of conventional anionic polymer dispersions in combination with active pigments or blocking agents, such as compounds of zinc, aluminium or titanium.2-4 Furthermore, it has been found that functionalities like urethane groups or alkyl-urea groups seem to interact with tannin, which leads to improved anti-bleeding properties of the base resin.2,5 Very recently, the use of amphiphilic block copolymers as tannin-blocking components for low-VOC coatings has been published,6 as well as waterborne emulsions based on solvent and plasticizer-free, anionic acrylic, core-shell copolymers.7
The objective of our work was to develop a one-pack, practically solvent-free cationic primer for wood that was fast drying and had good barrier properties on tannin-rich woods. The primer's nicotine blocking properties on concrete were also evaluated.

Cationic Waterborne Primer Synthesis
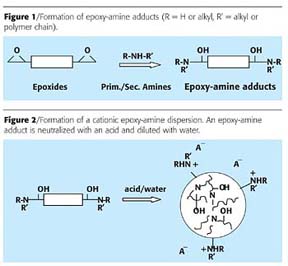
The first step in making cationic dispersions was to react epoxy resins with amines, as shown in Figure 1. Ring opening of epoxy resins with primary and secondary amines leads to an oxirane-free polymer network, which contains tertiary amine groups and secondary hydroxy groups. These basic tertiary amine groups in the polymer are intended to interact with the acidic tannin and should, therefore, contribute significantly to the barrier properties of the applied film.By varying the stoichometric relationship between reactants, the manufacturing process and the kind of raw materials used, one is able to design a water-dispersible polymer within a certain range of molecular weight, together with a proper mix of hardness and flexibility.
The epoxy-amine adduct was then neutralized with an organic acid in order to make it water dispersible. Water is added under stirring, whereby a cationic dispersion is obtained (Figure 2).
Because a process solvent has been used in this first synthetic approach, the final dispersion still contained a considerable amount of an organic solvent (Table 1).
For testing, one layer of the unpigmented cationic dispersion, 300 µm wet film thickness, was applied on Merbau wood. After a drying time of 24 h at ambient temperature, one layer of a waterborne white acrylic topcoat (300 µm wet film) was over coated and allowed to dry for 24 h at ambient temperature. The state of the coating was evaluated after being put into a humidity chamber for 5 weeks at 40 °C and 90% relative humidity.

As our goal was to make low-solvent cationic dispersions, we saw no possibilities to further increasing molecular weight with the applied technology, due to the high viscosity that occurs during synthesis.
Dynamic viscosity of the reaction mixture is the limiting factor here for manufacturing. Simply varying the stoichiometry of the reactants cannot help. Therefore we tried to further increase molecular weight in the dispersion state, rather than in the resin phase, where dynamic viscosity should not depend anymore on the molecular weight of the polymer.
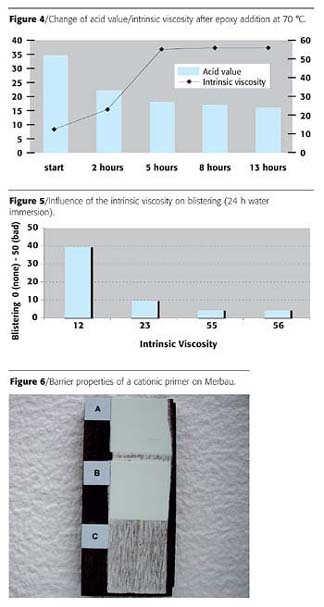
For this purpose, a cationic dispersion with an intrinsic viscosity of about 12 was heated to 70 °C and a portion of a liquid epoxy resin (5% on total solids) was added under good stirring. Table 2 illustrates the evolution of some key properties.
The breakdown of dynamic viscosity, from not measurable to very favourable values (Table 2), is interesting. Dynamic viscosity does not depend anymore on the molecular weight of the polymer, but only on the physical state of the dispersion.
It can be clearly seen from Table 2 and Figure 4 how intrinsic viscosity (and molecular weight) increases after epoxy addition. GPC analysis of the polymer indicates a molecular weight of ~ 2,500 (Mn) and ~ 45,000 (Mw) respectively after 13 h (Table 3). This also shows the effect of the "epoxy treatment" of the dispersion on molecular weight increase/distribution, when compared with the preceding values.
Also blistering after immersion of coated panels in water depends strongly on the intrinsic viscosity of the polymer (Figure 5 and Table 3).
Coating Application and Testing
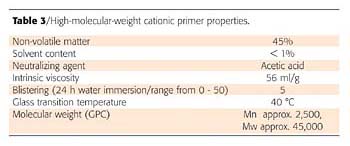
The above-described high-molecular-weight cationic primer was tested both as a barrier primer on tannin-rich wood (Merbau) and on nicotine stained walls.The binder was applied with 80 µm and 300 µm wet film thickness on Merbau and over coated after 24 h with a standard acrylic white, waterborne deco paint (300 µm applicator). It could be seen from prior evaluations that the best anti-bleeding properties are achieved by the resins alone as clear coats and that pigmented barrier primers, if not properly formulated, even performed less effectively.
After 24 h drying at room temperature the wood panels were put into the humidity chamber at 40 °C and 90% relative humidity. After 5 weeks in the humidity chamber no bleeding could be observed, whereas the topcoat applied directly on Merbau showed severe discolouring right after drying (Figure 6).
The evaluation of the barrier properties on nicotine stains was done in a similar way (Figure 7). A brown solution of cigarette contents was prepared by boiling 20 cigarette stubs in 100 ml of water ("Nicotine solution"). This solution was applied by brush on a white-painted concrete panel. Then the unpigmented cationic primer was applied by brush on the brown-stained surface. After 24 h drying, a standard acrylic white, waterborne deco paint was applied by brush. After 24 h drying at room temperature, the concrete panel was put into the humidity chamber at 40 °C and 90% relative humidity. We could see that the cationic dispersion itself showed some discolouring after drying (field 3), which however did not affect the topcoat. After 5 weeks in the humidity chamber no discolouring of the white topcoat could be observed (field 5), whereas the directly on the spoiled surface applied top coat showed severe discolouring right after drying (field 6).

Conclusion
New high-molecular-weight, epoxy-based waterborne cationic primers can effectively prevent topcoats from tannin and "nicotine" staining. High molecular weights have been achieved by the "epoxy treatment" of an intermediate epoxy-amine dispersion.
General properties like fast drying, hardness, good sandability, hydrolytic stability and good adhesion on different substrates (i.e., concrete, wood, metal, old alkyd paint, etc.) can make them useful as "universal primers".
References
1 Ekstedt, J., Studies on barrier properties of exterior wood coatings. Doctoral Thesis, 2002.2 Loos, F. et. al., Waterborne binder composition with Zink complex. PCT/EP01/06461, 2001.
3 Rota, D. et. al., Primerless latex paint with tannin blocking. US 6,531 223 B1, 2003.
4 Staffel, T. et. al., Titanium chelate additives for use in water-soluble polymers. PCT/EP02/02208, 2002.
5 Twene, D. et. al., Waterborne unique binder for Zink-free extrudates blocking applications. Pitture e Vernici, European Coatings 80(3), 2004.
6 Kimerling, A.S., et. al. Block copolymers as low-VOC coatings for wood: Characterisation and Tannin bleed resistance. Progress in Organic Coatings, 51(1), 2004.
7 Solich, A., New waterborne emulsion for stain blocking primers. Pitture e Vernici, European Coatings 79(10), 2003.
This paper was presented at the 8th Nürnberg Congress, Creative Advances in Coatings Technology, April 2005 in Nürnberg, Germany. The Congress is sponsored by FPL, PRA and the Vincentz Network.



Report Abusive Comment