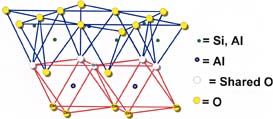
Structure
The kaolinite structure can be depicted as a layer of silica rings joined to a layer of alumina octahedra through shared oxygens, as shown in Figure 1. A well-formed individual kaolinite particle has the shape of a hexagonal plate. In nature these plates occur in stacks or "books" that exhibit varying degrees of stacking regularity. Because an individual kaolinite particle has an oxygen surface on one side and a hydroxyl surface on the other, it is strongly hydrogen bonded to the plates above and below it. This makes delamination more difficult than for the other platy silicate fillers, talc and mica. Figure 2 shows platy kaolin particles after delamination. Kaolin is hydrophilic and thus readily water dispersible; for nonaqueous applications, matrix compatibility is improved by surface treatment.
Kaolin Grades
Nearly all of the coatings-grade kaolin currently produced in the United States is sedimentary clay from Georgia, which hosts most of the world's major kaolin belt, stretching the 250 miles between Aiken, SC, and Eufala, AL. Georgia's clay reserves are estimated at 1.4 billion tons. Despite the well-entrenched position of the kaolin belt producers, a source of naturally high-purity, high-brightness kaolin has recently come on stream in Utah.1Kaolin clay is commonly differentiated as "hard" clay or "soft," according to terminology borrowed from the rubber industry. Hard clay is relatively poorly crystallized, very fine-grained kaolin, about 0.2 to 0.4 æm median particle size by sedimentation. It provides reinforcement in rubber, resulting in hard, uncured compounds. Soft clay is a better-crystallized, coarser kaolin, about 1.3 µm median particle size by sedimentation. It has a low reinforcing effect in rubber, resulting in softer uncured compounds.
The basic differentiation of coatings-grade kaolins is hydrous clay and calcined clay. Hydrous clays are structurally unmodified, retaining their hydrophilic surface hydroxyls, the so-called water of crystallization. Most of the hydrous clay used in coatings is water-washed. Water-washed grades are made by slurrying the clay in water and then centrifuging or hydrocycloning it to remove impurities and produce specific particle size fractions. The refined slurry is either dewatered (to reduce soluble impurities) and dried, or concentrated to 70% solids and sold in slurry form. Water-washed clays are often treated to improve their brightness. This includes chemical bleaching and/or high-intensity magnetic separation to remove iron and titanium impurities.
Delaminated clay is made by attrition milling the coarse clay fraction from the water washing of soft clay. This breaks down the kaolinite stacks into thin, wide individual plates, improving brightness, opacity and barrier properties.
Airfloat clay is dry-ground hydrous kaolin that has been air-separated to reduce impurities and control particle-size distribution. The coatings industry uses only minor amounts of air-float clay because of its generally poorer color and its greater abrasivity, caused by mineral impurities, compared to water-washed grades.
Calcined clay is made by the thermal treatment of water-washed and bleached kaolin. Low-temperature calcination, at about 650-700 deg C, removes structural hydroxyls and forms amorphous metakaolin. Specific gravity is reduced from 2.58 to about 2.50 in the process, while hardness and porosity, and thus brightness, opacity and oil absorption, are increased. Fully calcined clays, with maximum brightness and opacity, are produced in the 1000-1150 deg C range. This is hot enough to totally collapse the amorphous structure, with a consequent increase in specific gravity to 2.6-2.7, without causing the mineralogical transformation to mullite (specific gravity 3.2, hardness 6-7). The balance of opacity and sheen derived from calcined clays can be manipulated by the temperature, rate of heating and fluxes used in the calcination process.
Because of the importance of kaolin's effect on the optical properties of coatings, these clays are offered in more varieties than are other silicate functional fillers in order to provide a range of particle crystallinity and shapes, controlled particle size fractions, brightness and opacification. These clays are made even more versatile through chemical modification. Grades are available with dispersant coatings for easy dispersion in water, as well as stearate or silane surface treatment for improved compatibility with organic matrices. "Structured" clay products, in some cases also called SAMS (synthetic alkali metal alumino-silicates), are made by reacting kaolin with alkalies, such as alkaline silicates, under elevated temperatures and pressures. The resulting alkaline products are essentially kaolin plates with a rim of amorphous reaction product. The reaction and subsequent agglomeration can be conducted to provide products with increased, but controlled, levels of porosity, oil absorption, brightness, opacification, tinting strength and flatting.2 A different approach to structured pigments for better optical properties is to electrostatically bind oxide particles, such as silica or titania, to clay faces.
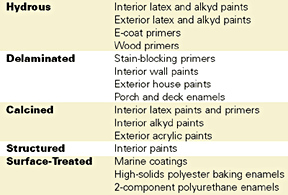
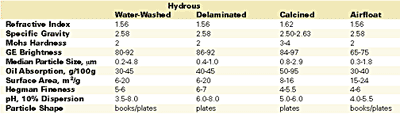
Properties and Uses
The United States is the leading supplier of filler-grade kaolin clays, currently producing about 7.5 million tons/year, of which approximately 90% comes from Georgia and 45% is exported. The domestic consumption of Georgia kaolin consists of approximately 64% water-washed, 15% delaminated, 11% calcined and 9% airfloat. About 66% of domestic consumption is used by the paper industry for coating (56%) and filling (10%); the second largest user is the coatings industry, with 6%.3 The overwhelming emphasis on clay for paper coating, and the attendant focus on optical properties, accounts for the wide variety of clay products available to the coatings industry.The range of properties provided by kaolin clay products is presented in Table 1. The primary use of kaolin in coatings is as a TiO2 extender in waterborne architectural paints. Calcined clays generally provide the best brightness, TiO2 extension and dry hide. Water-washed and delaminated grades also contribute to extension and dry hide, as well as covering power and gloss control (finer particle size = higher gloss). The typical preferred uses by function of the various clay products are given in Table 2.
One author recently discussed fine particle size, 0.8æm and 1.4æm, structured-surface calcined kaolins that were designed for flatting in flat and satin finish paints without sacrificing opacity. These high-brightness products contribute sufficiently to dry film opacity that a significant reduction in TiO2 is possible, although in practice this is limited by the lack of a commensurate improvement in wet film opacity. In addition to sheen reduction with improved opacity, significantly improved touch-up properties were also noted.4 In the same article, an ultrafine, 0.2æm hydrous platy kaolin was described as an extender for gloss paints. Because this clay is similar in size to TiO2, it functions as an effective spacer, optimizing opacity by maximizing the exposure of TiO2 surfaces to light and allowing an 8-12% replacement of TiO2.
Beyond the Light
Despite the fact that the coatings industry focuses primarily on the optical properties of kaolin clays, this mineral also serves as a functional filler. Kaolin is an inherently fine-particle-size, platy, chemically inert mineral. It is available with neutral to slightly acidic pH and in controlled particle size distributions. Hydrous clays are used for their contribution to suspension stability, flow properties, leveling, film smoothness, film strength, and weatherability. Delaminated clays are preferred for barrier properties and for more controlled chalking and overall durability in exterior coatings. Because of their greater hardness, calcined clays provide better scrub resistance, which is otherwise not improved with kaolins. The types of coatings in which kaolin clays are used are indicated in Table 3.For example, an ultrafine hydrous clay used for efficient TiO2 extension has been reported to also improve exterior durability as well as gloss and gloss retention.5 Other applications where kaolin clays have been cited as functional additives are anti-corrosion paints, high solids paints, moisture-cured systems and automotive electrodeposition primers.6 In addition, a surface-treated, fine-grind kaolin has been shown to provide better chip resistance than mica, talc and barite in an intermediate coating.7
For more information, contact RT Vanderbilt Co., phone 203/853.1400; fax 203/853.1452; visit www.rtvanderbilt.com; e-mail pciullo@rtvan derbilt.com or srobinson@rtvanderbilt.com.
References
1 Utah Clay Technology, Kaolin Overview, www.utah.com, March 2003.2 Broom, T.T. Origins of Certain Kaolin Pigments and Their Relative Performance in Flat Wall Paints. Mod. Pt. & Ctgs. January 1997.
3 Virta, R.L. Clay and Shale, Minerals Yearbook; USGS, Reston, VA, 2001.
4 Ashek, L. New Generation Kaolin-based Pigment Extenders. PCI, March 2003
5 Irvine, E. No Compromise: The Effectiveness of Specialty Ultrafine Kaolin Pigments as TiO2 Augmenters. Mod. Pt. & Ctgs. January 2001.
6 Stoneback, C. Using Kaolin as a High Performance Coating Additive. PCI, October 1996.
7 Khokhani, A. Surface-Treated Aluminum Silicate can be Used in Industrial Coatings. PCI, February 1993.

Report Abusive Comment