Color generated by thin film interference can be likened to the colors observed from a soap bubble. Analogous to the film of a soap bubble, a metal-oxide film interacts with light, causing reflection of certain wavelengths. By carefully controlling the thickness of the film, the observed specular reflection is of a particular color. For TiO2, which itself absorbs no visible light, a white reflection is observed from the thinnest film, followed by gold, red, blue and, finally, green reflection from the thickest film. For iron oxide, which has a reddish orange color due to light absorption, the observed reflections begin with gold. At non-specular angles, the absorption color is observable and becomes stronger as the film thickness increases.
If metal oxide films are applied to a very small and/or irregularly shaped substrate, reflections will effectively be "scattered" over a range of viewing angles. The observed color at any one angle will then be too weak to observe. This is why such pigments inherently require a large flat (platy) surface - 5-50 mm, and commonly about 20 mm across. In addition, application methods that align platelets in a common direction maximize the special effect obtainable from a pearlescent pigment.
The acceptance and popularity of pearlescent pigments are due in large part to their appearance and performance. These are the result of the inherent lightfastness of TiO2 and iron oxide and the humidity resistance achieved by proper post-treatment. Historically, post-treatment has been with metal hydroxides, most commonly chromium (III) hydroxide.1-2
As coatings systems have evolved and waterborne systems have become more prevalent, the utility of chromium (III) hydroxide has declined. Coatings systems span a broader range of chemistries, and chromium is no longer universally successful. Additionally, the performance requirements have been raised for many applications, and metal hydroxide surface treatments are no longer capable of satisfying additional performance requirements. Lastly, chromium, even in the (III) oxidation state is of environmental concern in some countries.
Theoretical
Experimental observations have provided some insights into why there is a need to enhance the stability of a mica pigment with a post-treatment. It has been useful to attribute weakness to three areas: the size of the pigment, its surface chemistry, and stresses in the film.The paint film is polymeric, composed of polymer domains that act as a unified layer, having both cohesive and adhesive strength. This unified layer can be disrupted by pigment particles, especially large ones, resulting in a weakening of the film structure.
The surface chemistry of the mica pigment can also play a role. It contains polar surface sites. Water is ubiquitous, small, polar and capable of significant hydrogen bonding. Water can readily enter the polymer film and form an attachment at the mica pigment surface. Additional water can enter and attach to the first water through hydrogen bonding.3 Continued accumulation creates a "wedge" of water and causes pigment-vehicle separation. Reversible or permanent whitening, blisters, and delamination may result.4
Another factor to consider is stress in the paint film.5 Stress can exist as residual stress from the curing process and as additional stress caused from swelling of the basecoat by water uptake.
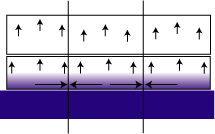
The residual or internal stress of a film is a result of the film's thermal history. When a cured film cools, the thermal expansion co-efficient difference between the film and the substrate is such that the film shrinks at a different rate than the substrate. The mica pigment can be considered to act as a substrate. Since the mica flake lacks the flexibility to deform, stress is created at the interface. In extreme cases, this can cause micro cracks and splinters, creating an entry point for moisture. When the film swells from water uptake, the mica flake's rigidity again becomes a factor. In an unpigmented film, when the coating swells, no stress is created because the forces point away from the substrate and are unrestricted (see Figure 1). The sideways forces push against equal but opposite forces from adjacent regions.6-9
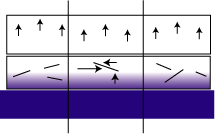
The presence of a large platy pigment results in additional shear forces along the platelet surface and twisting of the platelet within the film (see Figure 2). Mica flakes can cause additional variations in the forces because they are water barriers. They can hinder the water's access to areas just below the platelet. This causes differential swelling and uneven forces within the film. Ultimately this can lead to buckling of the basecoat (DOI loss).

Silane coupling agents are known to improve adhesion in many coating systems, and have been found effective for mica pigments.13-14 The most common coupling agents are tri-alkoxyl, silane alkyls, with the alkyl group ending in one of a variety of functional groups. The alkoxy groups are most commonly either ethoxy or methoxy.
(CH3CH2O-)3Si-(CH2)nR
(CH3O-)3Si-(CH2)nR'
Examples of the alkyl-functional moieties are two- or three-carbon chains ending in amine, isocyanate, epoxy, or methacrylate. In the presence of even trace amounts of water, the alkoxy groups hydrolyze, and will leave, for example:
(HO-)3Si-(CH2)nR
The individual units react with any available sites, including surface oxides and hydroxyls, as well as with each other. With each other, polymeric units are created, where one to three -O- or -O-H-O- linkages link two Si atoms. Additionally, the R group can undergo various acid-base or hydrolysis reactions, creating linkages with either an SiO-, the -R of another silane molecule, the -R' of another coupling agent, or metal hydroxide surface sites. These are random events, with populations of the various "possibilities" depending on the exact conditions (concentration, temperature and pH).
For the case of an aluminum hydroxide and a silane, the reaction scheme is shown in Figure 3.
Various combinations of mica pigments, metal hydroxides and silanes have been tested. Evaluation of the post-treatment for humidity resistance was based on appearance and performance (DOI retention and adhesion) in a waterborne basecoat/clearcoat paint system.
After extensive experimental work, the following generalizations can be made.
- Coupling agents coated directly onto metal oxide surfaces are less successful.
- Coupling agents introduced with metal hydroxides present are more successful.
- Combinations of coupling agents are better than a single coupling agent.
- Too much coupling agent causes pigment-pigment agglomeration.
- Curing of the silane coupling agent is critical.
- Some coupling agents are more effective than others.
Experimental
Current automotive OEM paint chemistry is specific and somewhat unique to each manufacturer, and is designed to meet the exacting requirements of each. In some circumstances adjustments are made for individual assembly plants. To simplify post treatment development a generic basecoat/clearcoat formulation was used specifically for mica pigments. The formulation practice and raw materials used were representative of those used throughout North America. European and Asian systems can vary somewhat due to environmental regulations and raw material availability. Performance in those systems will not be discussed here.The different surface treatments were compared in the following samples.
Samples A - D: TiO2 coated mica with:
- A - no surface treatment
B - chromium hydroxide treatment
C - TiO2 silane treatment
D - alternative silane treatment
Samples E - H: Fe2O3 coated mica with:
E - no surface treatment
F - chromium hydroxide treatment
G - silane treatment
H - alternative silane treatment
Each panel was evaluated before and 20 minutes after humidity exposure for changes in DOI (distinctness of image) and color. The exposure used a Cleveland condensation humidity cabinet following ASTM D 4585 for 240 hours at 40 DEG C. Fifteen minutes after exposure, a crosshatch adhesion test was done according to method B of ASTM D 3359.

Results and Discussion
The most common humidity-induced film failures for mica pigments are wrinkling, whitening, blistering and adhesion loss. These failures will greatly affect both appearance and structural integrity of the film.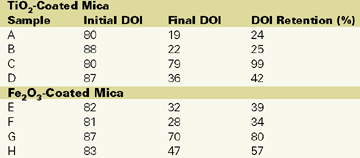
Appearance
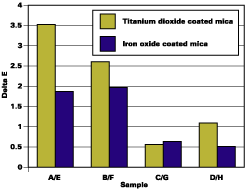
Whitening is caused by water uptake in the film. Micro water droplets have a different refractive index and scatter light, leading to a white or cloudy appearance. Whitening can be measured with DL from the CIELAB scale (ASTM D 2244). For mica-based pigments in automotive coating, whitening is the major component of the color change (DE).Table 1 shows results for whitening. The most change (4.14% and 4.46%) is observed for the untreated pigments (A & E). Chromium hydroxide treatment slightly reduces the whitening of the film. Optimal silane treatment minimizes the change, to 0.75% (C) and 1.69% (G). Figure 4, that plots DE change, shows the same trend.
An important aspect of the appearance of a glossy coating surface is the clarity of images reflected by them. Pockets of water within the film swell the basecoat more than the clearcoat resulting in micro-blistering and wrinkling. These failures can be measured with DOI of the coating surface (ASTM D 5767), which records changes in surface smoothness of the film.
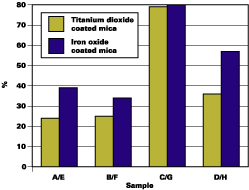
Figure 5 and Table 2 indicate the effect of surface treatments on DOI retention. There is a definite drop in DOI retention for chromium hydroxide treated material (B and F) as well as the untreated material (A and E). The basecoat with optimal silane-treated mica pigments (C and G) exhibits significantly less wrinkling and blistering than other treatments.

Structural Integrity
Because of the flake nature of mica pigments, when water gets into the film, it often causes delaminating of the pigment-binder interface within the basecoat, which contains mica pigments.
Adhesion is one of the key properties investigated in this study. Based on this evaluation, silane treatments perform much better than either chromium hydroxide treated or untreated material. For both titanium dioxide and iron oxide coated mica pigments, crosshatch adhesion data is presented in Table 3.
Conclusion
Data has been presented for performance after humidity exposure for characteristic mica pigments. The data demonstrates that the combination of metal hydroxide and silane coupling agent provide the highest performance enhancement for mica pigments.When comparing the pigments with chromium hydroxide treatment against those without treatment, a slightly better performance was found under extreme humidity conditions, such as less whitening and DE change. However, DOI retention and adhesion stays the same.
Comparison of silane treatment against the alternative silane treatment shows better film properties for DOI retention, which represents much less fading and wrinkling on the coating's surface after 240 hours of humidity exposure and equal properties for adhesion
In summary, surface treatment of mica pigments effectively improves film properties. The recently developed treatment significantly enhances the humidity resistance for waterborne coatings. It also offers an attractive combination of better performance and environmental compliance.
Acknowledgments
The authors wish to thank James Christie, James Carroll, Susan Rivera, Arthur Mason and the staff of Engelhard Corp.'s laboratories for their assistance with this article.This article was originally presented at the International Waterborne, High-Solids, and Powder Coatings Symposium February 6-8, 2002, in New Orleans
For more information on pigments, contact wei.liu@engelhard.com; or Circle Number 123.
References
Rieger, C.J.; Armanini, L. Cr(III) Treatment on Exterior Grade Titanium Dioxide Coated Mica, USP 4,134,776.Jackson, J. Nacreous Pigments Treated with Methacrylatochromic Chloride For Improved Humidity Resistance, USP 3,832,208.
Thiel, P.S. New Chemical Manifestations of Hydrogen bonding in Water Adlayers, Accts Chem Res., 24, 1991, 31-35.
Nguyen, T.; Bentz, D.; Byrd, E. A Study of Water at Organic Coating/Substrate Interface. J. Coatings Technol., 66, 1994, 39-49.
Farris, R.J.; Goldfarb, J.; Maden, M. The Correlation Between Residual Stresses and Delamination Behavior of Polymer Coatings. Makromol. Chem., Macromol Symposium 68, 1993, 57-69.
Vrtisand, J.; Farris, R. Experimental Stress Analysis Methods and Some Thin Film Applications. Material Research Society Symposium, 338, 1994, 527-540.
Taylor, J.; Tong, Q.; Farris, R. Effect of Ultraviolet Curing Temperature on the State of Stress and Mechanical Properties of Photoresists. SPIE 1925, 457-467.
Perera, D.; Oosterbroek, M. Hygrothermal Stress Evolution During Weathering in Organic Coatings. J. Coating Technol., 66, 1994, 83-88.
Perera, D.; Eynde, D. Moisture and Temperature Induced Stresses (Hygrothermal Stresses) in Organic Coatings. J. Coating Technol., 59, 1987, 55-63.
DeLuca, C., Jr. Light and Moisture Resistant Metal Oxide-Coated Mica Pigments, USP 5,091,011.
Nitta, K.; Watanabe, T.; Suzuki, I. Water-Resisting Nacreous Pigment and Process for Producing the Same, EP 0 268 918.
Sullivan, W.J.; Cacace, D. Improved Weather Resistant Pearlescent Pigments, USP 5,432,912.
Venturini, M.; Lavallee. C.; Cacace, D. Pearlescent Pigment for Exterior Use, USP 5,759,255.
Duschek, J.; Glausch, R. Pearlescent Pigment for Water-Borne Surface-Coating Systems, USP 5,472,491.


Report Abusive Comment