
Polyester degradation is affected by solar radiation, photocatalytic admixtures, water and moisture, chemicals, oxygen, ozone, temperature, abrasion, internal and external stress, and pigment fading.3 Out of all these, the following factors, all present in outdoor weathering, are the most important to coating degradation: moisture, elevated or lowered temperatures, oxidation and UV radiation.
Most plastics are generally evaluated in three conditions of elevated-temperature exposure: elevated temperature over a long period of time, elevated temperature over a short period of time, or cyclic exposure to elevated and lowered temperatures, such as may occur during alternating day and night exposure.2
When temperature is increased, the destructive effects are further enhanced by additional UV exposure.
This process is sensitized by the presence of chromophores, such as carbonyl (-C=O) containing compounds.1 By the absorption of photons (hn), the sensitizer energy is raised to an excited state; in this excited stage it may split, producing free radicals. These free radicals will then react with the surrounding oxygen molecules, forming peroxide or hydroperoxide radicals - radicals that can further attack other polymer chains, perpetuating the reaction. The oxidative degradation of polymers is catalyzed by heavy metals.4
Different stabilizers may be used to reduce polymer degradation: antioxidants, thermostabilizers, photostabilizers, etc.
The photochemical effect of sunlight on a plastic material depends on the superficial absorption properties and the chemical bond energies of the material. Light wavelengths that have the most affect on plastics range from 290 to 400 nm. The wavelength of UV radiation whose photon energy corresponds to a particular bond energy in the polymer chain (see Table 1) can break the chemical bonds (through a chain scission), changing the properties, and therefore the performance of the polymer.6 The most damaging wavelength for polyesters is believed to be 325 nm.2
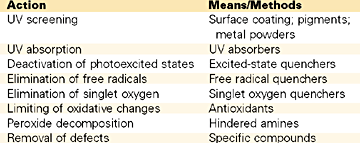
Theoretically, there are four different chemical methods that can be used against plastics deterioration.6 The first technique is to introduce additives that absorb light before they can contact the degradation-initiating center and the energy is dissipated as heat.
The second way consists in deactivating the excited state of the initiating center. Again, such reactions produce energy, which is generated either in the form of heat or as fluorescent radiation.
In the third method, the hydroxyperoxides that have been formed are transformed into other stable forms that do not generate free radicals. Metals complexes of sulfur-containing compounds are very efficient in this regard and can be effectively used at low concentrations. Dialkyldithiocarbamates, dialkyldithiophosphates and thiobisphenolates are used for this specific purpose.
The fourth method is achieved by introducing in the system new additives (free radicals scavengers) whose purpose is to stop further reaction of the formed free radicals, by simply reacting with them and forming a stable compound. The hindered amine light stabilizers (HALS) most likely operate by this chemical mechanism. Beside HALS, nickel complexes, which are considered excited state quenchers, are also being used.
However, the addition of UV absorbers alone may not always give adequate protection to all type of plastics; generally they are used in combination with HALS compounds.8
The degradation process mostly affects the surface, at least in the initial stages, and results in chalking, discoloration and sometimes even loss of physical and electrical properties. The product bulk properties of impact strength, tensile strength and elongation may all deteriorate because of degradation process.
Wypych8 also describes the sequence of events during degradation and the results of exposing coating to weather, such as chalking, discoloration or cracking. He considers gloss to be the most sensitive measure of deterioration because the degradative process affects the surface first, followed then by the color change.
Any specific polymer may have good resistance to a particular weathering factor and may be highly susceptible to degradation when exposed to other conditions or a combination of factors.9 For example, higher temperatures will increase the oxidation rate of a plastic, as well as the rate of photochemical reactions. Exposure to lowered temperature can cause a material to become brittle; the modulus of the material will be higher, while the elongation and impact resistance may decrease. Crazing, cracking and propensity to fracture upon impact can also occur. When plastics are exposed to elevated temperature, the result may be loss of mechanical properties (mostly embrittlement, loss of impact, flexibility, and elongation) or loss of electrical properties.9 During the cyclic exposure, the alternating expansion and contraction of the plastic will eventually lead to mechanical fatigue failure and cracking. It can also result in electrical failure due to the formation of surface crazing, that may subsequently be filled by moisture and dirt, forming a conductive medium that promotes electrical tracking.
This article evaluates the influence of several UV inhibitors on the gelcoat performance and properties. It also compares the most common types of accelerated weathering devices with natural weathering test results.

Experimental
The gelcoat is a very important part in composites and needs to perform two primary functions - protection against weather and decoration.8,10 The need for weathering studies became obvious when the gelcoat business realized that the gelcoat durability can be severely affected by the coating degradation during exposure. To predict the behavior of the plastic materials in different weather conditions prior to its field use, test methods designed to simulate natural weathering at an accelerated rate have been developed. Due to these tests, the long-term weathering performance may be predicted in a shorter time period by exposing specimens to extreme conditions to accelerate the aging process.
While the real-time outdoor weathering exposures provide the most accurate results, they are extremely slow and accelerated testing methods were therefore needed to reduce the cycle time associated with new product development, and to have a consistent way to compare performance. Unfortunately, not all accelerated weathering tests' methods or equipment accurately predict the relative durability of the coatings because it is almost impossible to rank the degenerative power of temperature, moisture and UV radiation or their combination.7

The type and degree of outdoor exposure vary greatly from place to place throughout the world, Florida and Arizona being international benchmarks for natural weathering. Florida has high intensity sunlight, annual UV and year-round temperatures, annual rainfall and humidity. Arizona features a hot, dry and high UV radiation environment, with large temperature fluctuations. With the exception of higher humidity, the Arizona exposure condition is a harsher climate. For our own real-life outdoor test, we have selected a method with a direct exposure on unbacked specimens on a wood fixture frame as per ASTM G 7-96, so that the front surface (gelcoat) of the panels faced the sun and had no cover.
Choosing a specimen exposure angle will also have a significant effect on each of the following test parameters: solar energy dosage, specimen temperatures and moisture and time of wetness.12 The angle of exposure, called "tilt angle," has a significant impact on the specimen's response to its environment because it determines the dosage of solar radiation, rate of heat build-up and cool down, and the amount of time that the specimen is wet because of dew formation, rainfall, or drying winds. For our test, we selected a 34-deg north latitude angle, where the specimens were mounted facing south. This exposure angle is known as "Latitude angle" and provides the most solar radiation of any fixed angle.

Materials
A special modified acrylated polyester gelcoat resin and a general-purpose (i.e., conventional) unsaturated ortho polyester resin for the base core were used for this study.The gelcoats and the core mix were formulated using the formulations shown in Table 3 expressed in weight parts per 100 parts of neat resin (phr). These formulations and the mixing conditions do not necessarily reflect practical requirements for the resin mixes.
For this study we selected two main types of light absorbers commonly used in the industry (a hydroxyphenylbenzotriazole and a hydroxybenzophenone), along with a HALS (piperidinyl sebacate), as shown in Table 4. The UV inhibitors were added only in the gelcoat mix in the amounts specified in Table 5.
Procedure
All the resin mix components were dispersed for 20 minutes in a mixer with a blade diameter of 50 mm. A shear rate of 7.8 m/sec (3,000 rpm) was applied, if not otherwise specified. With the exception of the type and amount of UV inhibitors in the gelcoats, the same single chemical composition was used for all the panels.A film thickness of 0.3 mm of gelcoat was drawn down on a horizontal surface and then cured in the convection type oven at 126.7 deg C for 20 minutes. A flat panel structure (1,500 gram per square meter total weight and 1.37 mm thick) was built together using the above gelcoat and the core, with 25 wt% total glass content. The whole composition was cured in the convection type oven at 126.7 deg C for 25 minutes.
The gloss was measured on the gelcoat side of the panel at 60 deg with a Byk-Gardner micro-TRI-gloss instrument, as per ASTM D 523-85 standard. The difference ("d" Gloss) between the initial and the final (after exposure) gloss was reported.
The color on the gelcoat side of the panel was measured with an UltraScan XE spectrophotometer from HunterLab. The CIE system of L, a, b values represents whiteness, redness, and yellowness, respectively, with the corresponding complementary colors at the opposite end of each axis of 3-D colorspace. The total color change, dE, was calculated after each exposure by the equation:
dE = ((dL)2 +(da)2 +(db)2 )
where: "d" represents the difference between the initial and final values on each color axis of arbitrary units.
The following accelerated weathering tests were performed on the panels: Emmaqua, Xenon arc, and UV, along with the real-life outdoor exposure.
The Emmaqua test is an accelerated natural outdoor weathering test performed in Arizona, where the solar radiation is magnified by a sun-tracking mirror device. A total radiant exposure energy of 280 MJ/m2, in accordance with ASTM G 90-94, Procedure B, is considered to be the equivalent of one year of real-life natural outdoor exposure in Arizona. The specimens are mounted unbacked in an aluminum frame, with the gelcoat surface toward the sun.
Xenon arc test exposure was performed as per ASE J 1960/ASTM G 26 standards, with an Atlas Ci65-A instrument.
UV exposure was performed with a Q-U-V Accelerated Weathering Tester instrument from The Q-Panel Co., with a combination of condensation and fluorescent UV, as per ASTM G 53. UV-B lamps were used on the gelcoat side of the panels with the following continuous cycle: 4 hours of UV at 50 deg C, followed by 4 hours of condensation at 40 deg C.

Results and Discussion
In the first study, as shown in Table 6, we evaluated the real-life weathering data (color change and gloss change), after one year of natural outdoor exposure. As can be seen, there is not much difference between the hydroxyphenylbenzotriazole and the hydroxybenzophenone; regardless of their amount, their performance is very much alike. Not the same thing can be said about the HALS: by itself, it does not offer any color change (yellowing) protection to the polyester gelcoat. The yellowing protection does increase with the amount of UV absorber(s), but the efficiency is not linear. The best results were obtained, however, with the larger amounts of additives and with the synergistic effect of both inhibitor(s)/HALS.
While the yellowing differences are considered important, one year of outdoor exposure is not long enough to evaluate the gloss retention. At this level, the differences reported between the systems are too small to be considered indicative to actual performance differences. It should therefore be considered as a fact that the color change does actually occur prior to chalking and loss of gloss.
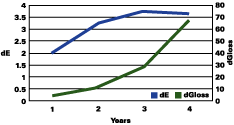
Figure 1 shows that the color change continued to rise up to three years, followed by a plateau, and even a decrease in the total color change. The gloss continued to decrease during the years. The color change "stability" seems to occur when the gloss has lost about 30 units.
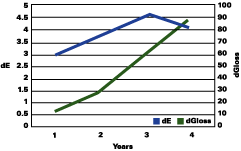
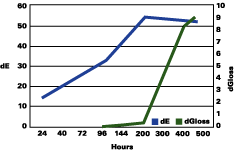
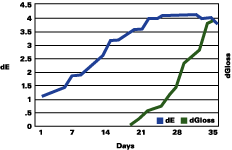
The data reported suggests that up to a gloss loss of about 30 units, the rate of yellowing is much greater than chalking; after that, the chalking prevails and the apparent yellowing is stopped and even reversed. Premature or very delayed chalking will drastically change the aesthetics of the gelcoat. The degree of curing of the resin, its chemistry, hardness and the thickness of the gelcoat become therefore very critical.
Conclusion
From a range of UV inhibitors, two UV light stabilizers and a HALS were evaluated in the polyester gelcoat of the FRP panels. Both the hydroxyphenylbenzotriazole and the hydroxybenzophenone evaluated in this study provided equal results. The total color change was significantly reduced when combined with the HALS. By itself, the HALS does not provide any surface stability improvement in the gelcoat. The best results were obtained with the highest amounts of inhibitors.To evaluate the gloss retention, more than one year of outdoor weathering is necessary. Due to the complex multi-process (photolysis, photooxidation, hydrolysis) nature of degradation chemistry, no theoretically based model exists to predict deterioration associated with weathering. There are however several proposed lab methods, which were tested in our evaluation. Each one can provide important information, but none actually can accurately match the real-life outdoor weathering data. In our proposed weathering model, we believe that the color change occurs first, followed by a gloss reduction due to chalking.
The evaluation of an UV inhibitor (or mixtures of inhibitors) against another UV inhibitor (or mixtures) becomes also even more challenging because of the specific spectral emission wavelength of each instrument, which, unfortunately, are different one from another and more importantly, than the solar radiation.
For more information on UV inhibitors, contact Lasco Composites, 8015 Dixon Dr., Florence, KY 41042; phone 859/371.7720; fax 859/371.1913; e-mail cezarcapanescu@fuse.net.
References
1. Seymour, R.B. Long-Term Environmental Factors, Eng. Plastics, Vol. II, (1988)423-432.2. Delre, L.C.; Miller, R.W. Engineering Plastics, Vol. II, (1988)575-580.
3. Ashbee, K. Fiber Reinforced Composites, Lancaster, (1993)317.
4. Seymour, R.B. Chemical Susceptibily, Eng. Plastics, Vol.II, (1988)571-574.
5. Nichols, J.W. Chemistry of Polymers, Cambridge, (1991)135.
6. Miller, E. Engineering Plastics, Vol. II, (1988)493-507.
7. Plastic Design Library, Effect of UV Light and Weather, NY, (1994),IV,V.
8. Wypych, G. Handbook of Material Weathering, Toronto, (1995)357-518.
9. Maccani, R.R. Engineering Plastics, Vol. II, (1988)68.
10. Nistor, C.; Ripszky, S.; Izrael, Gh. Materale Termorigide Armate, Bucuresti, (1980)108.
11. Crump, L.S. Durability of Gelcoats, CI 51st Conf., (Feb.1996)3.
12. Supplier, Q-LAB Tech. Bulletin LL-9025, (1998).


Report Abusive Comment