The coatings industry has come a long way from brushes and buckets. Over the past 30 years, chemical engineers have formulated innovative polymer resins that have a range of modulus (from high to low), better fire retardancy, low VOCs and faster reaction times. Today's modern coatings, including polyurethane and polyurea, are among the most advanced resins developed. These polyureas, more than any other polymer coating, stand out in their versatility, strength and longevity. They are the next step in the coatings evolution.
At the same time, advances in coatings technology have also led to the development of spray, injection and roto-cast application equipment that also improves the performance of polyureas. In particular, plural component equipment technology has been improved to make spray application easier, more uniform in coverage, applicable at lower pressures (as low as 1,000 psi) and generating less overspray or fogging.
Polyurea materials have two components: the isocyanates quasi-prepolymer and a resin blend. The resin blend is a combination of amine-terminated chain extenders. Unlike polyurethanes, no polyols are used in the manufacture of polyurea resins. When the materials are mixed together in the application equipment, the isocyanates and the amine resins react to form a urea linkage (see Figure).
Introduced in 1989 by the Texaco Chemical Co., polyurea was regarded by many in the coatings industry as an "over-hyped" product with exaggerated features and benefits. True, polyurea systems and technology have many outstanding properties. However, many manufacturers of traditional coating material discounted the claims of polyurea being the "wonder" product; as a result, many manufacturers and end users lost sight of the true advantages of the product.
Many old-guard coatings manufacturers did not differentiate between polyurethane and polyurea. All coatings, whether polyether amines (polyurea component) or polyester/polyether hydroxyl (polyurethane urethane component) resins, were identified as polyurethanes. Only in the past seven years have many companies differentiated these coatings.
OEM manufacturers, contractors, engineers and fabricators needed a fast-cure, moisture-insensitive coating system. They required a variety of physical properties, excellent adhesion, smooth surface flow out, superior tensile strength and high abrasion resistance (see Table). A polyurea system fit that description. The main physical properties of polyurea explain their success.
| Fast Reaction Time |
Polyurea's fast reaction time (5-15 seconds) leaves polyurethane and epoxy materials in the proverbial dust. It is an autocatalytic polymer. With the fast reaction time, polyureas do not easily react with humidity and moisture in substrates, so the material can be readily applied over cold or damp substrates, such as steel, concrete, wood or PU foam. On the other hand, when smoother flow of material is required to wet out a mold or cover an intricate carved surface, polyureas (like polyurethanes) can be slowed down to have gel times ranging from 20-60 seconds.
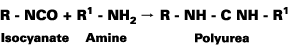
|
| Figure 1 |
Fast reaction time is a great advantage of polyurea. In the case of facility maintenance or rejuvenation, owners want to regain usage of the facility as soon as possible. A fast-reacting polyurea fully cures within a few hours, whereas most polyurethane or epoxy coatings require 24-48 hours before the coated areas can be used to their full potential.
Polyurea is frequently used to protect the exterior of iron pipe, since the pipe can be recoated "above-the-ditch" (in the field) without expensive equipment. The polyurea cures quickly and the pipe can be almost immediately reburied without crack. Many pipelines around the world, including the Trans-Alaska pipeline, have used this technology to speed production and keep maintenance costs to a minimum.
Moisture Insensitivity
Where moisture or humidity is a concern, polyureas outperform any product on the market today. Polyurethanes can be sensitive to high humidity and moisture in a substrate. As a result, they will react with atmospheric moisture or high humidity to produce carbon dioxide gas and cause foaming and/or pinholing in the surface. In contrast, polyureas are not affected by moisture.
The reaction of Component A and Component B (polyamines) or a polyurea system is so fast that the moisture reaction can't occur. Therefore, the polyurea systems are not sensitive to moisture and humidity, and do not normally produce carbon dioxide bubbles. This is an advantage in climates or regions with high humidity or dewpoints.
On the whole, polyureas are a more versatile protector for concrete. Recent innovations have led to the blending of epoxy and polyurea to form materials that are ideal for below-grade waterproofing, bridges, parking decks and flooring. Since these are slower curing systems, aggregates such as stone, iron or quartz flakes can be added to improve traction for vehicles and non-skid qualities for pedestrians.
Excellent Adhesion
If a substrate is moist or has condensation on it, polyurea will perform much better than polyurethanes. Many in the industry were surprised when Texaco produced a video of polyurea being sprayed over ice and water without affecting the reaction of the components. This demonstration is generally not encountered in real-world applications. Applying any coating over an excessively wet or unprimed/unprepared substrate can have detrimental effects on the adhesion.
Organizations such as the National Association of Corrosion Engineers and the Steel Structures Painting Council issue guidelines for properly preparing and priming surfaces. A properly prepared surface will improve adhesion, especially for critical applications such as moist concrete for containment lining and flooring, as well as adhesion protection for geotextile, wood and steel. In these applications, polyurea systems will have the best results.
The heat sink typically causes application problems for steel substrates. Conventional polyurethane systems applied to heat sink substrates could compromise the properties or adhesion of the first pass. There are no problems using a polyurea system over prepared steel.
Superior Tensile Strength
In waterproofing applications, a low modulus and a high elongation elastomer are required to meet the challenge. Polyurethanes have traditionally been regarded as having higher elongation and a lower modulus than a polyurea with a similar hardness. However, the days of polyurethanes having lower modulus than polyureas are over. Modern advances in the chemistry mean polyureas are formulated to feel as soft and elastic as polyurethane. This, in turn, opens the door for OEM manufacturers that produce soft "vinyl-like" skins, for example, to choose polyurea. The new polyureas will stretch with much less force. And, more importantly, polyureas will resist punctures and tears equally as vigorously as polyurethanes.
Low to No Volatile Content
The composite and OEM fabrication industry has seen a dramatic jump in the use of polyurea as an alternative to unsaturated polyester vinyl esters and polyester fiberglass for open mold part production. Automotive and off-highway body panels, bumpers and other parts are just a few of the parts that can easily be manufactured more quickly and without harmful emissions. Many manufacturers in the marine, bath fixture and recreational vehicle industry can also turn to polyurea as a reliable, cost effective alternative to FRP systems. The low volatile content of polyurea also makes it very attractive for confined workspaces. Many local and state government agencies are cracking down on styrene emissions. Unlike polyester fiberglass or epoxy, no fumes or styrene emission are associated with polyurea. Therefore, polyurea is ideal for applications such as wall and floor coatings for the food and beverage industries.
Until recently, manufacturers have been faced with the choice of shutting down production or, in the case of large companies, paying the fines and continuing with production in hopes there is enough profit margin in each unit produced to keep the company operating. Modern structural polyurea systems can offer these companies an alternative production material to eliminate government fines and increase profits.
High Abrasion Resistance
In a highly abrasive environment, polyureas perform extremely well. In applications once thought to require high-solids epoxies, polyureas are winning at every turn. In the rail and barge industry, polyureas are used instead because of their superior elongation and high impact resistance. Epoxies will crack and delaminate when exposed to constant pounding.
The durability and physical properties of polyurea have made it the system of choice for the rapidly growing truck bed lining industry. Polyurea is sprayed in a uniform texture over the metal bed surface. Plus, thanks to innovations in application equipment, non-professional sprayer and retail applications can quickly turnover multiple trucks in an afternoon. Polyurea has proven its distinct advantages over polyurethane liners.
Heat and Fire Resistance
When it comes to heat resistance and fire retardance, polyureas have the advantage over comparably formulated polyurethane. Because of its formulation, structural/rigid polyureas have excellent resistance to heat distortion and sagging. At the same time, polyurea maintains its flexibility and high impact resistance. This is not the case for molded structural polyurethane parts that are in constant close proximity to heat. Polyurethanes tend to sag. Polyureas resist heat sag and maintain their shape.
And when the heat is really on, like in the case of fire, polyureas will naturally outperform most other polymer resins. The resulting low smoke and flame spread is due to polyureas' molecular structure. Exposed to constant flame for 20-30 seconds, polyurea will self-extinguish.
Long-Term Stability
Many polyureas are based on aliphatic isocyanate prepolymers that are highly weather resistant and color stable. Products based on aromatic isocyanate prepolymers are not color stable and will tend to chalk or darken in color with extended exposure. For a long time, aromatic polyurea was the primary version of polyurea that was promoted to the industry. In the mid-1990s, aliphatic and aliphatic-modified polyureas were developed. These products revolutionized the coatings business. Now end-users could safely choose a polyurea for applications that would be constantly exposed to sunlight, without fear of discoloration and/or chalking.
Environmental Protection
Exposed polyurea is widely used in applications such as concrete or geotextile coatings for secondary containment applications. Polyureas' fast cure times allow it to be rapidly applied to a prepared substrate with minimal downtime for the facility. This has made polyurea the choice of facility managers for walls around and floors under chemical storage of diluted acids, alkali, salt solution, organic solvents and oils. Polyurea provides a strong barrier to spills from reaching the environment. In this type of application, polyurea readily conforms to footings, pipes and protrusions to form a complete seal. An aliphatic polyurethane or epoxy topcoat can be added for aesthetics in environments that are too highly corrosive for a standard polyurea.
Conclusion
In a world of increasing environmental awareness, polyurea proves to be an effective and economical choice for governments and businesses for their elastomeric and structural needs. Material improvements in cure times, hardness and fire retardancy are being made everyday. Application equipment and spray tip innovations are being introduced more rapidly than ever to meet the demand for better, more efficient means of getting the product sprayed in place.
Polyurea Protects Rail Cars from Corrosion
The area in and around rail cars is one of the most unforgiving places for any coating. Iron and steel impacting each other can quickly turn a seamless epoxy coating to dust and start the cycle of corrosion. Also the constant loading and unloading of cars filled with coal, lumber, steel and grain creates a highly abrasive environment that only the toughest coatings can withstand.
In highly abrasive situations, elastomeric polyureas are outperforming traditional paints and epoxy coatings in physical properties and economics. As the cost of repairing and maintaining rail cars continue to rise, rail car owners and transit authorities are turning to high performance protective coatings to keep their rail cars in operation as long as possible.
In one instance, Specialty Products Inc. (SPI), Tacoma, WA, was chosen by the U.S. Department of Energy (DOE) to supply a coating for the interior of 30+ rail cars. But these cars were not going to carry the average payload. The project called for these cars to contain and transport low-level radioactive material through the United States. Other spray polyurea coatings had been considered but SPI's performed best for this application.
The payload transportation would be challenging enough. Now the application time would stretch the limit of any elastomeric coating. All 35 cars in the project needed to be prepared, coated and readied for transport in just 28 days with restrictions on the operating hours mandated by the train yard official. Every step of the project would offer a challenge to overcome.
The specification called for a polyurea coating that did not require a primer. The polyurea coating had to have excellent adhesion to the steel and be able to withstand the abrasion of load shifts as the cars rumbled down the line. Furthermore, even though the steel substrate of the rail cars had numerous imperfections, the surface of the coating had to be 100% free of pinholes and flaws. Any holidays in the coating could lead to failure of the lining and a release of the low-level radioactive materials. The call went out to Dan Helton, president of Specialty Products, for a solution. Helton recommended SPI's Polyshield HT, a spray-applied, two-component pure polyurea coating. The coating would be sprayed at a minimum thickness of 60 mils (1.5mm). The special formulation of Polyshield HT for direct to metal applications has a high pigment content terra cotta color with SPI's Adhesion Enhancer #4.
According to Dick Hugo of Applied Surface Technology Inc., the contractor for the project, "The coating was a perfect choice. It could be quickly and easily applied in the train yard with Gusmer(r) heated plural proportioning equipment. We bought equipment directly from Specialty Products. It helped having a one call source for the material and equipment."
The area in the rail yard designated to spray was tented off to prevent overspray from drifting to other cars. Hugo added, "To meet the deadline we literally had to line up the rail cars and start spraying." One by one, the steel surfaces of the cars were blasted with a three-mil (0.075mm) anchor profile. The polyurea was applied between 60-80 mils (1.5-2 mm).
"Specialty Products provided us with a coating that had slower than normal gel time to produce an exceptionally smooth surface and enhanced adhesion," he said.
Normally, polyureas are known for their extremely fast gel times (as little as five seconds) to prevent ambient or surface moisture from affecting the coatings. In these circumstances, the surface of the coating tends to form an "orange-peel" surface. However, polyurea can also be engineered to gel more slowly. As a result, the coating has time to flow out and form a glass-smooth surface.
According to John Hall, a project manager for the U.S. Department of Energy at the Fernald site, "We're very impressed how well polyurea is holding up over time." He continued, "The polyurea coating was robust in the field. It adhered very well." The material has remained in place through the sizzling summers and frigid winters of southwest Ohio.
Hugo credited the success of the job to the right coating and an exceptional crew of workers. Specialty Products provided five days of on-site training for the contractor.
Boston Transit (the rail company moving the material), the Department of Energy and the Department of Transportation inspected the final project. The direct-to-metal polyurea had to withstand a 1,750-psi pull-off test. The polyurea passed with flying colors. In the end, not one rail car had a defect in the surface. The cars were completed within a few hours of the deadline.


Report Abusive Comment