The earlier paper identified three primary requirements for effective systems of this type: 1.) the coating should have high wetting properties in order to penetrate existing rust layers at power cleaned areas, 2.) it should efficiently inhibit the progress of corrosion at these same areas, and 3.) it should accommodate incurred stress accumulation without undergoing brittle failure (i.e., without failing adhesively or cohesively itself and/or without inducing similar failures in the pre-existing aged oxidizing coating system to which it is applied).

Coating System Design Resolutions
It was suggested that failure propensity might be reduced by attention to the design of the coating system, rather than the formulation of the mastic itself. This might be achieved by restricting application of recoats only to those aged systems that are in good condition, and which were originally applied over well profiled (blasted) steel where the adhesion of the old system to the steel would be maximized.Where the epoxy mastics must be applied over old oxidizing systems, themselves applied over old mill scale, or where the condition of the original substrate is unknown, stress induced failure could be mitigated by minimizing film thickness and/or the number of coats applied over the old film. Alternatively, it was theorized that maximum corrosion protection over now rusted areas might be achieved by maximizing film thickness and/or the number of coatings applied over the rusted steel. In the first place, delamination would be minimized by maximizing adhesion and/or minimizing strain accretion in the new (and, therefore, in the existing) composite, without compromising the protection of areas still bearing viable lead/oil paint. In the second case, protection against corrosion could be upgraded by maximizing the total barrier over the vulnerable corroding areas where the original system had been lost.
In all events, the lead pigment is never atomized, and is encapsulated beneath the new working composite.
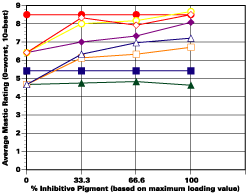
A. 5% Salt Spray Evaluation
Mastic Formulation Resolutions
It was suggested that rusting and delamination may be avoided by addressing aspects of the aluminized epoxy mastic formulation that also related to the maximization of wetting and corrosion inhibition, as well as the mitigation or avoidance of stress.
The severe delaminations that have haunted this type of system are clearly stress induced and are believed by these authors to be caused by the imposition (especially the sudden imposition) of hygrothermally applied tensile stress to an already strained system (under an internal strain residual from film formation). Formulation options for the mitigation of these failures were considered most importantly to be found in binder design although the use of platy pigments such as aluminum, along with the proper selection and optimization of corrosion inhibitors and extenders, may act as stress dissipation centers.
Barrier Primers, Inhibitive Primers and the Effect of Depassivating Salts
For many years, corrosion protection of steel by coatings has been attributed to one of several mechanisms: cathodic protection with zinc systems, the inducement of passivating layers on the steel beneath the coating by employing inhibitive pigments, or barrier techniques using electrolytically resistant binders of low permeability giving maximized oxygen deprivation at the steel surface and high resistance inhibition.2 Since the establishment of below film passivity on the metal requires at least some degree of water access into and through the film to the metal, and barrier techniques involved a mitigation of transmission of ionic solutions, if not exactly a suppression of water transmission, these two routes to the same end seem somewhat incompatible.3
While both techniques depend on the restriction of the influx of depassivating ions from outside the film to the metal beneath the film, in the case of the passivating type system, the presence of passivating water soluble materials within the film necessary for the consequent development of passivity beneath the film, inevitably compromises the high electrolytic resistance at the interface on which barrier protection is thought to largely depend. The use of soluble passivating materials such as chromate pigments within the film, will also give rise to osmotic blistering under conditions of high humidity and/or fresh water immersion.4 This will also eventually weaken performance.
In spite of these things, in private studies, it has been shown that certain inhibitive pigments of low solubility appear to be not only compatible with barrier pigments such as aluminum flake, but deliver some form of synergy. For many years a major producer of aluminum pastes successfully marketed a paste inhibited with strontium chromate. In the authors' laboratories, good performance with little or no osmotic blistering has also been achieved using certain modified zinc phosphates of low solubility with aluminum flake in epoxies and in moisture curing urethanes.
Formulation Design
It seemed important to investigate the use of similar pigment combinations in aluminized epoxy mastics designed for use as corrosion resistant overcoating paints to be used over both power cleaned rusted (and heavily pitted) steel, as well as over well blasted steel. The effects of formulation on stress mitigation and delamination are also critical and have pertinence to corrosion protection. After delamination occurs corrosion will freely occur, irrespective of the designed-in effectiveness of the newly delaminated coating. Stress induced delaminations of these systems will be investigated separately and discussed in a separate paper.In the current work, two types of mastics were considered, both based on a low molecular weight liquid epoxy resin (EEW 188) modified with about 12.7% by volume of a non-reactive non-volatile liquid hydrocarbon plasticizer designed to secure better rust penetration and adhesion over rusted surfaces. Alternative curing agents were employed in these studies, an unmodified cycloaliphatic amine and a proprietary polyamide modified with a small amount of cycloaliphatic amine. The latter curing agent had secured a good performance history in epoxy maintenance systems for all types of steel surfaces. Both curing agents were employed at true stoichiometry with the epoxy resin.
In all the materials tested, 24.2% non-leafing aluminum flake on total pigment volume was employed. This equated to about 4.9% aluminum volume on total volume of the dry film or 8.3-8.6% by total weight of dry paint. In these systems, three inhibitors were evaluated, a commercial strontium zinc calcium phosphosilicate (Halox SZP-391), a proprietary enhanced zinc ortho phosphate complex (Halox CZ-170), and an experimental organically modified calcium borate (Halox OMCB) designed for overcoat or higher film build applications.
Each of these inhibitive pigments was used at volume loadings of 5.4%, 3.6%, 1.8% and 0% on total dry paint volume. In these systems, an amino silane surface treated calcium silicate based auxiliary, found to be of value in other epoxy systems, was used at loadings 0%, 1.8%, 3.6% and 5.4% (replacing each inhibitor on a volume basis). Total pigment volume devoted to the inhibitive/auxiliary package was thus 5.4% of total dry volume, (except in the non-inhibited control paints 84 and 85). Talc and barytes were also included as inert extenders to make up the required pigment volume. All coatings were made on a high-speed disperser, with aluminum flake being incorporated at lower speed on a lightening mixer after dispersion of the other pigments. Paints were allowed to stand for three weeks prior to mixing and application. A 45-minute induction time was allowed between mixing of components and application, in order to avoid any possible amine blushing. Formulations were balanced with a mixture of xylene and a high boiling naphtha to 68% solids by volume and to a VOC level of approximately 270-280 g/l. Additional volumes of xylene were added to each formulation to facilitate spray application. This reduced volume solids to approximately 60.5% and elevated VOC to about 340 g/l. Controls based solely on aluminum with both inhibitor volume and calcium silicate volume replaced by additional barytes (Paints 84 and 85) were included using both curing agents. Formulations for the work are presented in Table 1. Alternative compositions based on the three inhibitors were otherwise identical; only the inhibitor weight changed as the weight per gallon of the inhibitor changed. Even though this is not the preferred method, inhibitors were exchanged on an equal volume basis to facilitate the ease of evaluation based on the formulation and project design. The slight differences in weight between the inhibitors is not deemed critical in this study.

Application and Testing Protocols
Films of all systems were applied to both sandblasted steel panels and to heavily rusted steel panels (cut from simple angle irons of previously exposed bracings). Applications were made by conventional spray using a 45-minute induction period after mixing. Coatings were applied in one and two coats, with film thicknesses determined using electronic gauges after first coats had dried and again after the final system had fully cured. Second coats were applied to half of the panels on each substrate after the first coat had dried for four days. Several test environments were selected in which to evaluate the mastic. These included 5% salt spray (ASTM B 117), continuous humidity at 50?C (ASTM 4585), prohesion (ASTM G 85-94 and XA5) and the ASTM D 5894 cycling exposure between prohesion (one week) and fluorescent condensation exposure (one week). UVA lamps were employed in the fluorescent condensation experiments, alternating four hours light at 60 deg C and four hours condensation at 50 deg C. Prohesion employed a one hour exposure to sodium chloride/ammonium sulfate electrolyte (0.05% and 0.35% respectively) at ambient temperature with a one hour dry off at 35 deg C.The temperature of the continuous humidity test (50?C), in hindsight, is thought to be too high, and may facilitate an unnaturally increased migration of the water into and through the film, especially where Tgs are lower than 50?C. This could inappropriately increase the tendency toward osmotic blister generation.
Either two or three panels of each mastic on each substrate in each test environment were tested, but only one panel of each mastic on each substrate was exposed in any given environment in any single group of panels i.e. at any one time. (Cabinet capacities required several identical but subsequent exposures of the duplicate groups of panels). On sandblasted steel, film thicknesses ranged from 2.1-2.9 mils (and averaged 2.43 mils per panel) for one coat systems, and from 4.4-5.5 mils (averaging 5.0 dry mils per panel for the two coat systems). Over rusted steel the film thicknesses on the one coat panels ranged from 4.6 to 5.5 mils (averaging 5.1 mils per panel) and on two coats from 6.2-8.0 dry mils (averaging 7.0 dry mils per panel). Film thickness determinations were standardized using appropriate shims, but great difficulty and uncertainty reduced accuracy over the rusted steel surfaces because of the heavy pitting.
After backing the panels with adhesive vinyl tape and scribing to the base steel with a tungsten carbide scribing tool, panels were evaluated for corrosion resistance and blistering resistance in each of the separate environments using the four different exposure protocols. A further series of panels were prepared for evaluation under practical marine exposure conditions at Kure Beach. While data on these panels are, at the time of writing (three years), not yet available, indications are that the data patterns correlate well with the overall findings from the present accelerated study, although the relative intensity of attack may vary. It is hoped that data from the exterior exposures at Kure Beach may be presented in a forthcoming paper.
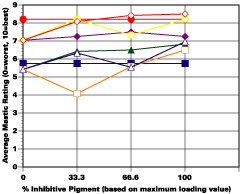
A. 5% Salt Spray Evaluation
Data
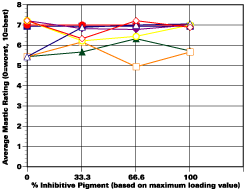
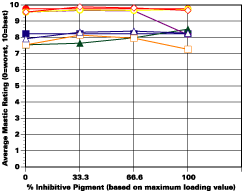
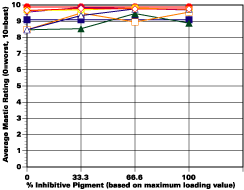
The study was unfortunately very large, and involved well over a thousand panels. In consequence, an in-depth presentation of all details is here precluded because of space. The effect of inhibitor loading in each of the four accelerated test environments employed may be reviewed in Figure 1 (for the cycloaliphatic amine cured systems) and in Figure 2 (for the cycloaliphatic amine modified polyamide systems). The overall value for performances is achieved by averaging the data for general panel corrosion resistance (ASTM D 610) and blistering resistance (blister degree and blister size - Federal Standard TT-P-141a Method 6461) with values for scribe corrosion resistance and filiform attack (ASTM D 1654), where these values were recorded (i.e., in the salt spray, prohesion and ASTM D 5894 data). The following digest of data is felt to accurately reflect the findings of the study, and the conclusions remain meaningful. Copies of a full transcript of the work may be obtained from Halox.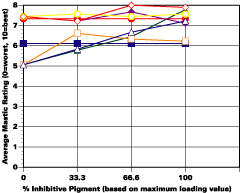
Number of Coats
Perhaps the most striking finding from these studies, was the poor performance realized from all mastics when used as single coats over rusted surfaces. Performances of simultaneously tested two-coated panels using the same mastics over the same type of surface was universally better. Excessive deterioration and obvious performance differences between the one and two-coated panels were most dramatic in salt spray, but were noted to a somewhat lesser extent in all three of the other environments. In the prohesion/QUV tests over these same rusted surfaces, differences between the performance on one and two-coated panels, were probably the least apparent, although the two-coated systems (with a single spurious exception) were almost universally preferred.
Panel performance over sandblasted steel was much better than over rusted steel. In this case, the value of a second coat of mastic was less easily established. Only with the non-inhibited controls based on the cycloaliphatic amine cured mastic, where corrosion was noted on the single panel systems tested in salt spray, was the advantage of a second coat clearly evident. In the ASTM D 5894 cycling test and in the prohesion test slight corrosion was noted on several single coat sandblasted steel panels that used the cycloaliphatic amine based coatings (including the inhibited systems). Here advantages were again realized from the use of a second coat.
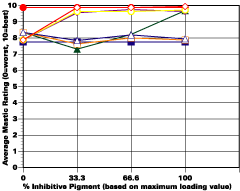
The Use of Inhibitors
Substantial performance enhancement was in most tests realized from the introduction of the inhibitor into the pigment mix, and maximized performance return was derived from higher inhibitive loadings (67-100% inhibitor). Positive effects were noted more with the polyamide cure than with the cycloaliphatic amine, and more in salt spray, prohesion, and in the cycling environment than in continuous humidity, where blistering, especially in the two coat systems, tended to diminish the performance of the inhibitive film most especially over rusted steel.The Halox organically modified calcium borate (OMCB) appeared to give the best data and its effectiveness was more reproducible from test environment to test environment than was the case with SZP-391 or CZ-170. The inhibitors appeared to be more effective where the films were employed as single coats, especially where single coats were applied over rusted surfaces. Two coat systems, including two coat systems over rusted steel, responded less favorable to inhibitor inclusion, although in this regard the OMCB would again seem to be more broadly effective than either the SZP-391 or CZ-170. All of the inhibitors effectiveness was limited in the two coat systems, although the data shows no significant performance impairment to be related to the inclusion of the inhibitors. It would seem the majority of the corrosion protection in this case is the result of the barrier properties achieved from the higher film build and film density of epoxy overcoat formulation.
The overall higher performance profile of the inhibitors in the thinner or one-coat systems, would point to a direct relationship between the film properties and the inhibitor chemistry employed. The mechanism of the inhibitors tested is one of direct anodic inhibition and they become active as the coating film breaks down and allows the ingress of water and aggressive electrolytes. The higher soluble OMBC pigment seemed to be more effective at controlling corrosion in the one coat or thinner film systems in comparison to the lower soluble SZP-391 or CZ-170. The unique performance seen with the OMBC inhibitor in thicker films can be tied to the organic modification and its affinity for promoting adhesion at the coating/substrate interface.
The inclusion of small amounts of the treated calcium silicate seemed quite justifiable of a cost effectiveness basis, although levels above 33% of the inhibitor/auxiliary volume are not recommended.
In general, the inclusion of a corrosion inhibitor was found beneficial to the corrosion protection of the coating. It is clear further optimization of the inhibitor/auxiliary pigment volume in relationship to the film thickness is necessary with this system.
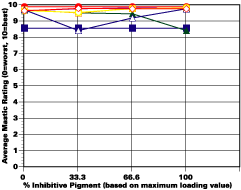
Osmotic Blistering and Effects of Inhibitors and Substrate
While no attempt was made to further increase the amount of inhibitor/basic auxiliary at the expense of the barytes or talc, the performance of two controls wherein the active pigments were entirely replaced with additional barytes, was in some environments (e.g., continuous humidity), surprisingly good, especially at high film thicknesses. This data seemed to support the original contention that barrier protection may be osmotically compromised by the inclusion of an active inhibitor. Even though they are relatively lower soluble type inhibitors, they will all contain some water of hydration that can cause the osmotic potential to be increased, especially in thicker more impermeable films such as the ones employed in this study. However, this was less of a detriment with the OMCB inhibitor possibly due to the modifications explained earlier. Over sandblasted surfaces, the non-inhibited system showed definitely improved blistering resistance compared to the inhibited offsets. The differentiation was more obvious in single-coated systems over sandblasted steel than over two-coated systems, which blistered more intensely than did the single-coated panels. This data is thought possibly related to the increased impermeability of the two-coated system and the more definitive membrane.The assignment of source for the osmotic driving force (inhibitor or the occluded salts in the substrate) that produces blistering in continuous humidity is important from a mechanistic point of view. Neither the one nor two coat films of those mastics that contained no inhibitor nor basic auxiliary (only inert extender) showed any great propensity to blister over sandblasted steel in continuous humidity, while many of the inhibited films over the same substrate did. This would seem to indicate that the osmotic force is derived from the inhibitive/auxiliary package itself and not from the salt nests on the rusted panels.
Supporting this conclusion were the lower values for soluble chlorides and sulfates found when the rusted substrates were boiled in distilled water. It is also reasonable to assume that in these tests, on these panels, enhanced blister resistance of the non-inhibited film can be attributed from the oxygen depletion based on the enhanced barrier properties of the film.
Selection of Curing Agent
Clear patterns of advantageous corrosion resistance performance from either one of the two curing agents were not established in this testing. However, the CZ-170 and the OMCB appeared to give the best overall performance in salt spray and humidity, in both the cycloaliphatic amine and the modified polyamide system. These differences appear to follow similar patterns over both sandblasted steel and rusted steel.Environment and Failure Mechanism
In general, the prohesion/QUV cycling environment was considered to be the least aggressive accelerated test in this test program, and required longer exposure durations for appreciable deterioration. 5% salt spray was the most demanding and produced the most extensive deterioration. In salt spray, the primary deterioration was via scribe attack, and, on the single coat systems over rusted steel, corrosion breakthrough of the general panel. General panel breakthrough was less dramatic in the other test environments, although it did occur. In prohesion over sandblasted steel, filiform corrosion attack was noted, though this did not occur when the substrate was rusted steel. The inclusion of inhibitive pigments tended to reduce the levels of filiform attack. Filiform attack was not seen in the other tests (including the prohesion/QUV cycling test). In humidity the primary mechanism of panel deterioration was blistering.Conclusion
The most telling conclusions from this series of experiments is certainly the absolute necessity of multiple coats over power tool cleaned rusted steel for good corrosion protection. Single coats appeared to encourage rapid corrosion breakdown on these panels, probably from incompletely coated peaks at the high points of the degraded steel surface. Two coats greatly diminished this type of attack. In spite of generally thinner films, similar differentials between one and two coats of the mastic on well prepared sandblasted steel were not nearly so much in evidence, although two coat protection (as compared to protection with a single coat) was still preferred.Significantly perhaps the corrosion of single-coated panels over sandblasted and rusted steel appeared to be diminished when the inhibitors were included in the pigmentation. In general, the solubility of the pigment appeared to have less negative effect than might have been predicted which was especially true with the Halox OMCB. However, the use of any of the three inhibitors included in these mastic formulations was seen to be a positive strategy. In this respect, the Halox OMCB (experimental organic modified calcium borate) appeared to be somewhat more effective than inhibitors of lower solubility (Halox SZP 391 and Halox CZ 170). In most environments, performance appeared to improve as inhibitor concentrations were increased towards a maximum. The presence of the basic auxiliary pigment, widely employed in epoxy formulations, appeared to be less effective in this study, although controlled levels (33% basic auxiliary/67% inhibitor) were often found to be effective, and would certainly assist in reducing the cost of the overall formulation.
The use of inhibitors in the mitigation of filiform corrosion, which showed up in prohesion (but not in the cycling environment) was also of value. Interestingly, filiform corrosion was not noted on the rusted panels (only on the sandblasted panels).
Osmotic blistering was seen on films of all mastics over rusted steel in continuous humidity. The effect was noted with both the pure cycloaliphatic amine and the modified polyamide. It was less problematic on sandblasted steel, especially in thin films applied in single coats. Significantly, osmotic blistering, which occurred in most inhibited systems used as two coat films over sandblasted steel, did not occur to the same degree with the non-inhibited systems. This suggests that soluble ions from the inhibitor rather than salt nests from the substrates are involved in the blistering. Fortunately the environment most suited to the generation of this effect (continuous humidity at 50 deg C) is not encountered under practical service conditions.
For more information on epoxy mastics, contact Steve A. Hodges, technical director, HALOX, 1326 Summer St., Hammond, IN 46320-2240; phone 219/933.1560; fax 219/933.1575; e-mail shodges@halox.com; or Circle Number 131.
References
1 C.H. Hare, "Preventing Overcoating Failures," JPCL, Vol. 14, #11, p. 50, November 1997.2 C.H. Hare, "Corrosion Control of Steel by Organic Coatings," Chapter 55, p. 1023 in Uhlig's Corrosion Handbook, 2nd Edition, Ed. by R.W. Revie, John Wiley & Sons Inc., New York, 2000.
3 W. Funke, "Towards Environmentally Acceptable Corrosion Protection by Organic Coatings - Problems and Rectification," J. Coatings Technol., p. 31, Oct. 1983.
4 C.H. Hare, "Blistering of Paint Films on Metal Part I: Osmotic Blistering," JPCL, Vol. 15, #2, p. 45, Feb. 1998.
5 H.H. Uhlig, "Organic Coatings," Chapter 15, p. 245 Corrosion and Corrosion Control, 2nd Edition, John Wiley & Sons Inc., NY 1971.


Report Abusive Comment