In an effort to further reduce urban ozone and air toxics, the EPA and state regulatory agencies continue to tighten the allowable VOC and HAP contents in a variety of coating categories. These stricter regulations are making it increasingly difficult for coating suppliers to offer compliant products that also meet their customers’ performance and cost requirements.
The following paragraphs describe several new tools for formulating ultralow VOC and HAP-free two-component (2K) urethane coatings that perform like their medium-solids counterparts.
1. ACRYFLOW™ P120, a solvent–free liquid acrylic polyol
2. Luxate® HA-500, a highly functional, low-viscosity isocyanate crosslinker
3. TBAc, tertiary butyl acetate, a non-HAP and proposed VOC-exempt solvent
VOC contents below 1.0 lb VOC/gal were achieved using a combination of these raw materials and other components to optimize coating performance and durability. Performance was achieved without using expensive crosslinkers, resins, solvents or reactive diluents.
Low-VOC Acrylic Polyols
The acrylic polyol is the most important component in a 2K urethane coating formulation. It has the greatest impact on the coating’s VOC content, appearance and durability. The resin composition and molecular weight are the most important variables in designing acrylic resins. Traditional acrylic polyols are based on hydroxyalkylacrylates and methacrylates, styrene, and alkyl acrylates and methacrylates. Styrene is often used to reduce cost and improve hardness and chemical resistance, but it is usually kept below 20 wt % to minimize yellowing and solvent demand (VOCs). Methyl methacrylate (MMA), isobornyl methacrylate (IBOMA) and tert-butyl methacrylate (TBMA) also give hard polymers. MMA is inexpensive but gives high-VOC polymers, whereas IBOMA and TBMA are expensive but give lower-VOC resins. Other acrylate monomers often used to soften the resin are butyl and 2-ethylhexyl acrylates and methacrylates.
Process and Monomer Selection
Lowering the polymer’s number average molecular weight (Mn) and/or polydispersity (Pd = Mw/Mn) is a common way to reduce solvent demand. Below 2,000 Mn, the polymer’s glass-transition temperature (Tg) starts falling below the theoretical Tg calculated using the Fox equation. This, in effect, reduces the hardness of the resin before it is crosslinked but maintains the final film hardness once crosslinking has occurred. This reduces solvent demand “in the can” without reducing final film performance.With traditional acrylic polyols, lowering the molecular weight (Mn) below 4,000 can decrease crosslink density and performance. This is because hydroxyalkyl acrylates and methacrylates have essentially the same reactivity as the other acrylate and methacrylate co-monomers. As the molecular weight is lowered, the probability that the polymer chain will contain only one hydroxyl group, or none at all, increases. Non-functional and mono-functional polymer chains plasticize the coating, effectively reducing its hardness, chemical resistance, and durability.
Acrylic polyol producers typically increase the amount of hydroxy-functional monomer in the high-solids polymers to overcome this drop in functionality.1 This is why most commercial high-solids acrylic polyols have hydroxyl numbers in the 140–175 mg KOH/g, compared to 80–120 for higher molecular weight, medium-solids resins. This comes at a price, however, as the demand for isocyanate crosslinkers increases with the polyol hydroxyl number, and the coating flexibility and impact resistance tend to decrease.
Lowering resin polydispersity, on the other hand, reduces the high molecular weight fraction in the polymer, which has a disproportionate effect on solvent demand. This is typically achieved by careful control of the polymerization temperature, the type and level of chain transfer agent used, and even by selecting the right monomers. For example, bulky monomers such as TBMA and IBOMA tend to give lower molecular weight resins with narrow polydispersities. Recently, we developed a series of acrylic polyols that use allyl alcohols as the hydroxy-functional monomers. This change in monomers required substantial changes in the polymerization process. However, this new process is ideal for yielding low-molecular-weight acrylic polyols with an even distribution of functionality and fewer non- or mono-functional impurities.
Hydroxy-functional allylic monomers behave as chain transfer agents, which significantly increases the amount of terminal OH functionality in the polymer. High end-group functionality improves crosslinking and the performance of the final coating. Allyl monomers are also much less reactive in free-radical polymerizations than the acrylate and methacrylate ester comonomers. Consequently, allyl monomers rarely react one after the other, which improves the distribution of OH functionality in the polymer chain. This helps create a regular, three-dimensional crosslinked network in the final coating.
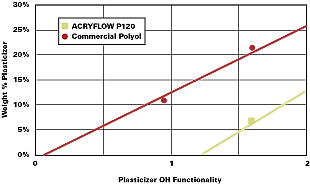
Figure 1 compares the OH number distribution as a function of molecular weight fraction for an ACRYFLOW resin compared to a popular high-solids acrylic polyol. The commercial oligomer had an average OH number of 145 g KOH/g, compared to 133 for the ACRYFLOW polyol. As discussed earlier, the higher OH number in the conventional polyol is required to compensate for the rapid loss of hydroxyl functionality that occurs as the molecular weight falls below 4,000 Mn.
Resin Selection
We have also discovered that combining two acrylic polyols, one with a low Tgand one with a high Tg, gives a system with lower solvent demand than having one resin with an intermediate Tg. We believe this is in part because the low-Tgpolyol acts as a reactive diluent and essentially replaces some of the solvent. Typical reactive diluents include low-molecular-weight polyesters and polyethers or more exotic chemicals such as oxazolidines. These diluents usually have much lower molecular weights and higher hydroxyl numbers than the primary resins. They often require higher isocyanate demand, and can decrease the coating’s performance.We have developed low-viscosity acrylic polyols that perform the same function as reactive diluents but without increasing cost, isocyanate demand, or significantly reducing the performance and durability of the final coating. Some examples of high and low Tg resins are listed in Table 1.
Another apparent reason this high/low Tg combination gives lower-VOC formulations is that lowering the molecular weight of high Tg resins has a disproportionate effect on their actual Tgs. For example, polyol “I” has a theoretical Tg of 75ºC but a measured Tg of 28ºC, a difference, or delta Tg, of –47ºC. A large negative delta Tg value is desirable. It shows that the polymer has high latent hardness but low solvent (VOC) demand. This is reflected in the low solution viscosities observed for these resins.
Isocyanate Crosslinkers Selection
The second most important component in a 2K-urethane formulation is the isocyanate crosslinker. The most common crosslinkers are the trimer and biuret of HDI (hexamethylene diisocyanate) and IPDI (isophorone diisocyanate). HDI trimer is probably the most commonly used because it combines low viscosity, intermediate cost, and gives coatings with outstanding properties and weatherability. HDI biuret is used in applications where cost is important and weatherability requirements are less stringent. Finally, IPDI trimer is often used in applications that require a quick physical dry such as automotive refinish applications. Even then, it is usually blended with HDI trimer to improve the coating’s flexibility and reduce cost.In an effort to further reduce VOCs, a number of lower-viscosity versions have been commercialized in recent years, including HDI dimer, low-viscosity versions of HDI trimer, and various allophonates. None perform as well as HDI trimer and all are significantly more expensive. However, we have recently developed proprietary, low-viscosity versions of HDI-trimer that perform as well and do not cost significantly more. Table 2 lists the physical properties of these new HDI-based crosslinkers.
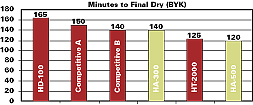
Unlike other low-viscosity isocyanate adducts, HA-300 and -500 dry quickly (see Figure 3) and give hard, yet flexible coatings like HDI trimer.

VOC-Exempt Solvents
It is a common misconception that solvents contribute little to a coating’s properties beyond the application stage. Solvents play an essential role in viscosity reduction but they also can affect the film’s dry time, appearance, adhesion to the substrate or undercoats, rheology, physical properties and weatherability.VOC-exempt solvents are particularly useful formulating tools because they require minimal reformulation and have less impact on the final coating properties. Unfortunately, there are only a handful of them, and most have significant performance or other limitations (see Table 4). Despite these limitations, acetone and PCBTF (para-chlorobenzotrifluoride) are commonly used in low-VOC coatings, especially those sold in California.
Acetone’s main limitations are its fast evaporation rate and extreme flammability. It is also an aggressive solvent, which can cause undercoats to lift, and is hygroscopic (absorbs ambient moisture). Hygroscopicity, and the rapid evaporative cooling that results from spraying coatings with high acetone contents, can cause hazing (“blushing”) in hot and humid conditions. On the other hand, acetone is very inexpensive, readily available, and a powerful solvent for most coating resins. It is, therefore, a useful “in-can” viscosity reducer.
Because of acetone’s fast evaporation rate, a slow or “tail” solvent must always be added for optimal coating appearance and performance. PCBTF is a slow solvent but behaves poorly as a slow solvent due to its low solvency power. Our attempts to use PCBTF as the tail solvent in zero-VOC, 2K urethane coatings gave films with poor appearance and performance. PCBTF also has a number of other properties that make it less desirable for compliant coatings. It is dense (11.2 lbs/gal), expensive, and malodorous.
Fortunately, the EPA has proposed to add TBAc to the list of VOC-exempt solvents and is close to publishing the final rule. TBAc is much less photochemically reactive than the solvents it will be replacing, such as toluene and xylene. Because it is a pound-for-pound replacement for these solvents in most formulations, TBAc can dramatically reduce the amount of ozone formed from coating operations. TBAc is also non-HAP, so the level of air toxics will also be reduced.
For the same reasons that TBAc has low photochemical reactivity, it is unusually chemically stable. Unlike other esters, it is resistant to acid or base-catalyzed hydrolysis and aminolysis. This makes it suitable for use even in strong acid-catalyzed baking enamels and 2K epoxy-amine coatings.4
Because of its intermediate evaporation rate and good solvency properties, TBAc can replace all of the acetone and PCBTF in current compliant formulations. When combined with an effective tail solvent such as PM acetate, MAK, NMP, or EEP it gives compliant coatings with outstanding properties and durability. Some examples are given in the following paragraphs.
Ultralow-VOC 2K Urethane Coatings
Performance requirements can vary greatly from one application to another but, in general, 2K urethane coatings must offer the following.- VOC and HAP contents low enough to meet regulations (below 3.5 to 2.1 lbs/gal)
- Reasonable cost and isocyanate demand
- A pot life of at least 2 hours
- Medium solids for ease of application (low viscosity)
- Sag resistance
- Fast physical dry (short dust-free time)
- Good flow and leveling (high distinctiveness of image or DOI)
- High gloss and clarity
- Hardness (pencil or pendulum)
- Flexibility and impact resistance (Mandrel bend and impact testing)
- Adhesion to substrates (crosshatch adhesion)
- Chemical and solvent resistance (MEK rubs and spot testing)
- Environmental resistance (gloss retention and yellowing index)
Optimizing one property often has a negative impact on another, so it is unusual to find a coating that scores high in all categories. Most often, one or more performance features are sacrificed at the expense of another. The coating properties most often sacrificed in high-solids coatings include the following.
- Pot life
- Ease of application
- Cost
- Appearance (especially flow and leveling and DOI)
- Weatherability
- Chemical resistance
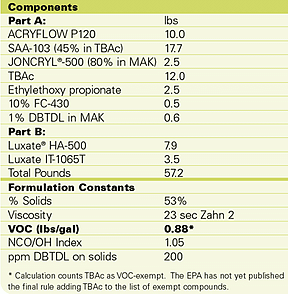
However, we have found that combining TBAc, high- and low-Tg polyols, and low-viscosity isocyanate adducts can give high-performance coatings with VOC contents at or below 1 lb/gal. These coatings were subjected to accelerated weathering tests and found to perform almost as well as the higher-VOC commercial systems in use today. The best formulation is listed in Table 5.
This clear coating was applied to a white, commercial automotive basecoat on a primed metal substrate at a thickness of 2.6 mil. The formulation’s pot life was estimated at 3 hours based on a doubling of the initial formulation viscosity. The BYK-Gardner final dry time was measured at 135 minutes. Film properties were measured after 336 hours and are compared to the best commercial benchmark we tested in Table 6.In summary, the ultralow VOC formulation gave a harder coating with physical properties and weatherability comparable to the best high-VOC benchmark. Only under harsh QUV-B conditions without UV-absorbers did the commercial system significantly outperform the ultralow VOC system. It did, on the other hand, yellow significantly more than the low-VOC system under the same conditions.
In other words, by combining exempt solvents and low viscosity resins and crosslinkers, it is possible to formulate solventborne urethanes with VOC contents below 1 lb/gal, lower cost, and performance equivalent to the best high-VOC solventborne systems.
Acknowledgements
The authors wish to thank Bob Good, Mark Smithson, and Dave Pangburn for expert technical assistance.For more information on additives, contact Lyondell Chemical, 3801 West Chester Pike, Newtown Square, PA 19073; phone 888/777.0232; fax 610/359.2841; visit www.lyondell.com.

Report Abusive Comment