- Molybdate corrosion inhibitors represent field-proven technology, demonstrated in numerous outdoor exposure studies and supported by over 30 years of commercial use in the industry.
- Molybdate corrosion inhibitors are effective in both conventional salt-spray tests and the newer cyclic salt-spray/UV exposure tests (e.g., ASTM D 5894) being used by the industry.
- Molybdate corrosion inhibitors are available in a range of pigment chemistries, thus allowing for more effective application in specific systems.
- Molybdate corrosion inhibitors are also now available in easy-dispersing, micronized grades, offering a finer, more narrow particle size distribution for optimized performance in thin-film applications (e.g., under 1 mil).
This article presents a review and update on molybdate corrosion inhibitor technology, with particular emphasis on recent testing of calcium zinc phospho-molybdate and easy dispersing, micronized molybdate inhibitors.
 Table 1 / Chemical and Physical Properties of Commercially Available Molybdate (Moly-White®) Corrosion Inhibitors
Table 1 / Chemical and Physical Properties of Commercially Available Molybdate (Moly-White®) Corrosion InhibitorsBackground
Molybdate corrosion inhibitors have been used in the coatings industry since the early 1970s. These pigmentary materials are produced using a patented process whereby active molybdate compounds (e.g., zinc molybdate or calcium zinc molybdate) are deposited onto relatively inert carrier pigments (e.g., zinc oxide or calcium carbonate) for optimized use of available molybdenum and reduced raw material costs.The protective action of molybdate pigments is based on the slight solubility characteristics of zinc and calcium molybdate compounds coupled with the well-recognized corrosion inhibiting action of molybdate ions in aqueous solution. When a coating film containing molybdate corrosion inhibitors is immersed or exposed to water, small quantities of molybdate ions will be released into the coating film. These molybdate ions will then interact with the metallic substrate to promote the formation of an adherent oxide layer, which prevents corrosion of the underlying substrate. This process is described as passivation.
Various product modifications in terms of chemistry, molybdate content and core pigmentation, have resulted in the availability of a range of commercially available molybdate corrosion inhibitors.
A series of phospho-molybdate pigments has also been introduced. These products were developed based on a synergistic action between phosphate and molybdate compounds in corrosion inhibition processes.
This range of molybdate pigments (see Table 1) has allowed for more optimized applications in specific types of coatings. Molybdate corrosion inhibitors are today used commercially by major industrial coating manufacturers in the United States, Europe, South America and Asia.
 Table 2 / Styrene Acrylic Latex Primer with Calcium Zinc Phospho-Molybdate
Table 2 / Styrene Acrylic Latex Primer with Calcium Zinc Phospho-MolybdateCalcium Zinc Phospho-Molybdate
The performance of the newer phospho-molybdate pigments was recently studied in the testing of a series of waterborne and high-solids coating formulations. In particular, calcium zinc phospho-molybdate was found to be a highly cost-effective and versatile corrosion inhibitor based on testing in the following systems. • Styrene acrylic latex primer • 2K waterborne epoxy primer • Water-reducible alkyd primer • 2K high-solids epoxy primerIn each of these studies, a model formulation (provided by the resin supplier) was used to evaluate the performance of calcium zinc phospho-molybdate vs. other corrosion inhibitors. Table 3 / Waterborne 2K Epoxy Primer with Calcium Zinc Phospho-Molybdate (4:1 mix ratio)The formulas for the calcium zinc phospho-molybdate based coating systems are shown in Tables 2–5. In all cases, corrosion inhibitors were tested and compared by substituting different inhibitors at an equal weight addition level in the formulas.
Table 3 / Waterborne 2K Epoxy Primer with Calcium Zinc Phospho-Molybdate (4:1 mix ratio)The formulas for the calcium zinc phospho-molybdate based coating systems are shown in Tables 2–5. In all cases, corrosion inhibitors were tested and compared by substituting different inhibitors at an equal weight addition level in the formulas. Figure 4 / 2K High-Solids Epoxy Primers after Accelerated Testing (substrate: hot rolled blast steel, dft: 3.0 mils, inhibitor loadings: 1.0 lbs/gal)Small adjustments in extender levels, to maintain equal PVC and volume-solids, were the only other differences between the formulations.
Figure 4 / 2K High-Solids Epoxy Primers after Accelerated Testing (substrate: hot rolled blast steel, dft: 3.0 mils, inhibitor loadings: 1.0 lbs/gal)Small adjustments in extender levels, to maintain equal PVC and volume-solids, were the only other differences between the formulations. Table 5 / 2K High-Solids Epoxy Primer with Calcium Zinc Phospho-Molybdate (4:1 mix ratio)Corrosion tests based on salt-spray (ASTM B 117) and cyclic salt-spray/UV exposure (ASTM D 5894) were then conducted to determine corrosion inhibitor effectiveness.
Table 5 / 2K High-Solids Epoxy Primer with Calcium Zinc Phospho-Molybdate (4:1 mix ratio)Corrosion tests based on salt-spray (ASTM B 117) and cyclic salt-spray/UV exposure (ASTM D 5894) were then conducted to determine corrosion inhibitor effectiveness. Figure 1 / Styrene Acrylic Latex Primers after Accelerated Testing (substrate: cold rolled mild steel, dft: 2.2 mils, inhibitor loadings: 0.5 lbs/gal)Representative results are presented in Figures 1–4, which show the appearance of test panels at completion of the corrosion tests.
Figure 1 / Styrene Acrylic Latex Primers after Accelerated Testing (substrate: cold rolled mild steel, dft: 2.2 mils, inhibitor loadings: 0.5 lbs/gal)Representative results are presented in Figures 1–4, which show the appearance of test panels at completion of the corrosion tests. Figure 2 / 2K Waterborne Epoxy Primers after Accelerated Testing (substrate: blast hot rolled steel, dft: 3.5 mils, inhibitor loadings: 0.5 lbs/gal)Market pricing information is included in the figures so that some appreciation of cost-effectiveness can be gained.
Figure 2 / 2K Waterborne Epoxy Primers after Accelerated Testing (substrate: blast hot rolled steel, dft: 3.5 mils, inhibitor loadings: 0.5 lbs/gal)Market pricing information is included in the figures so that some appreciation of cost-effectiveness can be gained. Figure 3 / Water-Reducible Alkyd Primers after Accelerated Testing (substrate: cold rolled mild steel, dft: 1.2 mils, inhibitor loadings: 1.0 lbs/gal)Calcium zinc phospho-molybdate clearly provides excellent cost-effective performance based upon these results.
Figure 3 / Water-Reducible Alkyd Primers after Accelerated Testing (substrate: cold rolled mild steel, dft: 1.2 mils, inhibitor loadings: 1.0 lbs/gal)Calcium zinc phospho-molybdate clearly provides excellent cost-effective performance based upon these results. Figure 4 / 2K High-Solids Epoxy Primers after Accelerated Testing (substrate: hot rolled blast steel, dft: 3.0 mils, inhibitor loadings: 1.0 lbs/gal)
Figure 4 / 2K High-Solids Epoxy Primers after Accelerated Testing (substrate: hot rolled blast steel, dft: 3.0 mils, inhibitor loadings: 1.0 lbs/gal)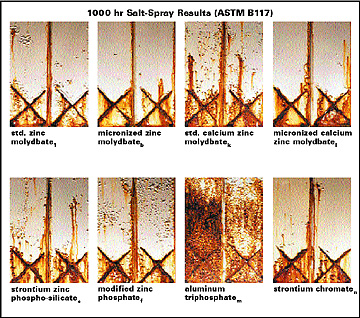 Figure 5 / Polyester Coil Coatings after 1000 hr Salt-Spray Test - Duplicate Panels (substrate: phosphated cold rolled mild steel, dft: 0.6 mils, inhibitor loadings: 0.67 lbs/gal)
Figure 5 / Polyester Coil Coatings after 1000 hr Salt-Spray Test - Duplicate Panels (substrate: phosphated cold rolled mild steel, dft: 0.6 mils, inhibitor loadings: 0.67 lbs/gal)Micronized Molybdate Corrosion Inhibitors
Molybdate corrosion inhibitors are now available in standard and easy-dispersing, micronized forms. The micronized grades offer a finer, narrower, particle size distribution allowing for improved ease of dispersion, increased surface activity and optimized performance in thin-film systems (under 1 mil dft).A recent study was conducted in a thin-film solventborne polyester coil coating formulation to compare the micronized vs. the standard molybdate pigment materials. Here, calcium zinc molybdate and zinc molybdate, in standard and micronized forms, were incorporated in a polyester coil coating formulation (provided by the resin supplier). Also tested were strontium chromate and a number of other inhibitors recommended by suppliers for thin-film polyester coil coatings.
The coil coating formulation based on micronized zinc molybdate is shown in Table 6. In all cases, corrosion inhibitors were substituted and tested at an equal weight addition level. Small adjustments in titanium dioxide levels were made to maintain constant PVC and volume solids.
Figure 5 shows the results of salt-spray testing, which is the most crucial corrosion test used by the coil coatings industry. The micronized molybdate pigments allow for performance characteristics comparable to strontium chromate, a corrosion inhibitor still widely used in coil coatings and aerospace primers despite serious toxicity concerns. Table 6 / Polyester Coil Coating with Micronized Zinc Molybdate
Table 6 / Polyester Coil Coating with Micronized Zinc MolybdateConclusion
The demonstrated performance capabilities of molybdate corrosion inhibitors, particularly calcium zinc phospho-molybdate and newly available micronized pigment grades, should allow for the continued growth of these important functional pigments.For more information, contact Moly-White Pigments Group, 601 Canal Road, Cleveland, OH 44113; phone 216/566.1294; fax 216/566.3837; e-mail chsimpson@sherwin.com; visit www.moly-white.com.
Industry Update: Molybdate Corrosion Inhibitors
In response to the ongoing elimination of chromate- and lead-based pigments and the need to identify effective replacements, molybdate corrosion inhibitors are finding increased application in the coatings industry. The key advantages associated with the use of these pigments as environmentally friendly corrosion inhibitors include the following.




Report Abusive Comment