
|
Over time, this art spread to China, India and Europe, where a trade was quickly developed.
It was soon realized that the small particles that were broken off the edges of the gold leaf during the hammering process imitated the gold leaf effect when incorporated in a suitable vehicle and applied on appropriate substrates. Hence the history of metal-pigmented ink and paint began.
The prohibitive cost of gold, however, limited the use of metallic coatings to jewelry, porcelain, chinaware and other art objects. Bronze and copper were soon recognized as substitutes for gold, and silver and tin were discovered to make silver shiny pigments.
It took until the middle of the 19th century to manufacture metal pigments on an industrial basis. At that time Sir Henry Bessemer developed the stamping process as the first practical and economical process for the manufacturing of metallic pigments.
Several years later, Charles Hall and Paul Herroult independently invented an aluminum smelting, which made aluminum more available. From then on, aluminum was adapted to the Bessemer process as a replacement for silver and tin. However, the drawback was that the process formed an explosive mixture with air over a range of metal-air ratios.
It took nearly 40 more years until E.J. Hall, in 1930, found a way to produce superior aluminum pigments under safe, explosion-free conditions: the wet ball milling, the final breakthrough in the industrial production of aluminum pigments.
|
| Click to enlarge |
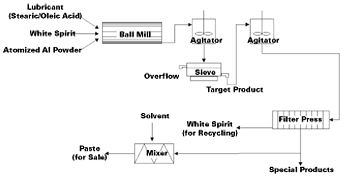
The Hall process (see Figure 1) is based on the milling of fine, spherical or irregular atomized aluminum powder (see Figure 2) in ball mills (see Figure 3) in the presence of steel balls, a suitable lubricant (normally stearic or oleic acid), and an aliphatic hydrocarbon, e.g., white spirit. The mills operate at a speed that allows the balls to cascade onto the powder and finally to flatten and break the flake.
The first generation of aluminum pigments resulted in ultrathin flakes with ragged edges and a cornflake-like appearance (see Figure 4).
In the following years, by close cooperation between pigment manufacturers, the ink and paint industries, designers, and stylists, the manufacturing process and thus the pigment quality was permanently optimized to produce a huge range of application-oriented pigments meeting nearly all requirements of the various industries.

|
| Click to enlarge |
The main requirements of the paint and ink industry were pretty similar: brilliance, lightness, hiding power and strong flop or two tone effect (angle dependent lightness of an application) combined with perfect nonleafing behavior, essentially a micro mirror.
A first step forward in the effort to improve the brightness and flop effect of those flakes was the development of the so-called “Silver-Dollar” pigments, showing greater particle thickness and more regular smooth rounded edges (see Figure 4).

|
| Click to enlarge |
Even with this development, not all of the requirements regarding brilliance and flop effect were fulfilled. The silver-dollar pigment was still a long way from providing the mirror-like effect that was desired.
The final breakthrough on the path to mirror-like pigments was described in 1976 in U.S. patent 3,949,139 and 1980 in U.S. patent 4,321,087. Both patents cover a method to produce fine mirror-like pigments in a physical vapor deposition (PVD) process.
The following sections offer a detailed description of those unique pigments, including their manufacturing process, typical properties and main applications.
PVD Aluminum Pigments (PVDA Pigments): The Manufacturing Process
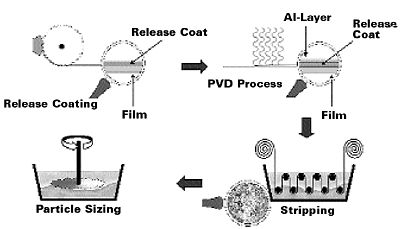
In a discontinuous process, a release coating (typical resin systems used are acrylics, cellulose, vinyl resins, etc.) is applied to at least one side of a carrier sheet by a coating or printing technique (preferably rotogravure or flexo). Suitable carriers are films consisting of polyesters, polyolefins or other common materials.
The coated film is then passed through a roll coater, where a very thin aluminum layer is deposited on the release-coat in a typical PVD process. The thickness of the deposited aluminum is crucial to obtain the desired properties of the final pigments. Usually, the thickness is adjusted between 30–50 nm. At a thickness above 50 nm, the orientation ability of the pigments is negatively influenced and the scattering effects at the edges of the pigments increase. Both effects have a negative impact on brilliance, opacity and flop. At a thickness below 30 nm, the aluminum becomes transparent and handling of the pigments is more difficult due to higher agglomeration tendencies.
In the next step, the metallized film runs through a solvent tank (stripper), where the release coat is dissolved and the aluminum layer is removed as fragments or coarse flakes. The solvent used in the stripper depends on the solubility of the applied release coat. Typical solvents are ketones, esters and alcohols. The aluminum fragments or coarse particles are washed and concentrated, if desired, to a dispersion normally containing 10–20% pigment. The particles are then sized by vigorous stirring or ultrasonic treatment.
These pigments are currently being offered to the ink and paint manufacturing industries as dispersions containing 10–20% aluminum pigment in various solvents, and are also offered as finished ink.
Properties of PVDA Pigments What Makes the Mirror-Like Metallic Effect
|
| Click to enlarge |
To understand the special mirror effects provided by these pigments, it is helpful to have a closer look at the principles of the metallic effect, how metallic effect is influenced by pigment properties, and what the major differences are between “conventional” metallic pigments and PVDA pigments.
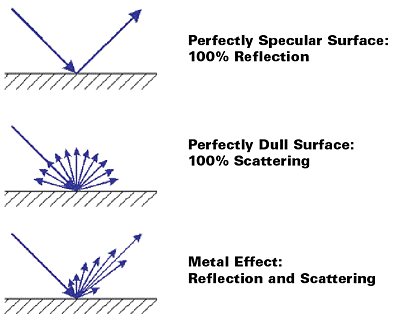
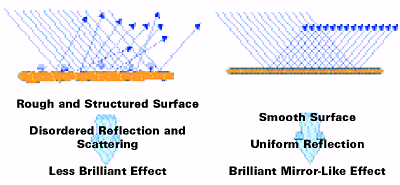
In the case of perfectly flat, high-gloss surfaces (e.g., mirrors), the law of reflection is valid: angle of illumination = angle of reflection. These types of surfaces are also considered image forming as the image of a reflected object can be distinctly seen. The light is reflected at the first surface into the specular direction of reflection.
In the case of perfectly dull surfaces, the light is scattered in all directions without a specular reflection. Metal pigments exhibit a combination of specular reflection arising from the (ideally) flat surface of the pigment and scattering caused by the pigment edges or defects on the pigment surface (see Figure 6).
To optimize the metallic effect, it is necessary to maximize the specular reflectance and to minimize the scattering.
|
| Click to enlarge |
Common discussions about pigment parameters that influence the metallic effect and how to improve this effect include particle shape (e.g., cornflake vs. silver-dollar), particle size, and particle size distribution. These considerations, however, are not very helpful in describing the difference between conventional flakes and PVDA pigments, as they are only valid when flakes that are manufactured according to the same technology are compared (e.g., comparison of fine and coarse particles manufactured according to the Hall process).
In the case of the pigments discussed in this article, these parameters are irrelevant as particle shape, size and distribution cannot be used to explain the mirror-like effect, and have little or no influence on the effect. Instead of this, two other properties are much more important: particle thickness and surface topography of the platelets.

|
| Click to enlarge |
Particle Thickness
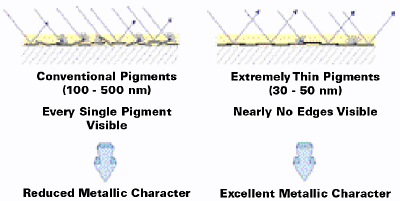
As mentioned, the thickness of PVDA pigments as compared to conventional flakes is approximately five to 10 times smaller (30–50 nm vs. 100–500 nm). This increases the mobility of the pigments significantly and allows them to orientate parallel to the surface of the substrate much faster than conventional pigments within a given time frame, e.g., during the short drying process of the ink or paint (see Figures 7–8).
The final orientation is only influenced by the topography of the substrate, the formulation parameters and the application technique but not limited anymore by the insufficient mobility of the pigment as is known from conventional flakes.

|
| Click to enlarge |
As demonstrated in Figure 7, optimized orientation results in improved brilliance, flop and coverage.
In addition, when viewing the top of a typical PVDA pigment application using an electron microscope, it is almost impossible to identify the edges of the pigments. Due to the extremely thin particles, the flakes form a nearly uniform surface without many visible edges where the light can be scattered. In comparison, applications with conventional pigments show the contours of each single pigment particle (see Figures 9–10).

|
| Click to enlarge |
Figure 9 demonstrates this effect which, in the case of very thin PVDA pigments, ultimately leads to an improved metallic character (high brilliance, good flop and coverage).
|
| Click to enlarge |
Surface Topography
Scattering and disordered reflection of light not only occurs at the pigment edges but also at defects on the pigment surface (see Figure 11).
Conventional pigments are exposed to strong mechanical forces during the milling process, resulting in uneven flakes with an irregular thickness and a large number of surface defects.
|
| Click to enlarge |
PVDA pigments, however, show a homogenous thickness and hardly any surface defects (see Figure 12), therefore guaranteeing a high level of specular reflectance. For these reason those pigments are often called “micro mirrors.”
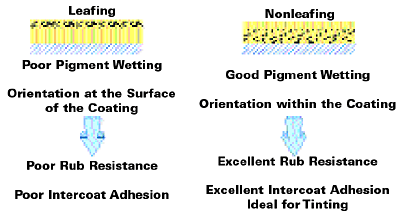
The Perfect Nonleafing Behavior

|
| Click to enlarge |
To complete this discussion of PVDA pigments, one further property is worth mentioning: the perfect nonleafing behavior (see Figure 13).
This property is generally known from conventional pigments and therefore not unique to PVDA pigments. However, this behavior opens up a range of formulation and application possibilities.
Applications and Formulations
|
| Click to enlarge |
The effects that can be achieved with those pigments not only depend on the typical pigment properties but are additionally influenced by other non-pigment-specific parameters such as formulation and application conditions.
The following parameters are generally known to support and guarantee the orientation of the pigments to ensure a high degree of brilliance, opacity, and flop. •
- Low-viscosity systems •
- Low binder contents or high pigment/binder ratios •
- Low pigment loading •
- Low film thickness •
- Smooth and even substrates
Unfavorable systems or conditions are therefore formulations with high viscosity or high binder content (e.g., high-solid paint systems, offset ink, screen ink, etc.), applications resulting in high film thickness (e.g., screen printing) and applications on uneven or absorbent substrates (e.g., prints on absorbent paper).
Graphic Arts Applications
|
| Click to enlarge |
Although this class of pigments was originally introduced to the paint market, the major market today is still within the graphic arts industry.
This market is mainly driven by the following three aspects. •
- In terms of metallic pigments by the slogan “brighter is better” •
- Ecological and recycling aspects •
- Cost efficiency
The pigments are mainly used as more cost effective and recyclable alternatives to metallized paper or film and foil stamping (see Figure 14). They add brilliant metallic effects to labels, folding cartons (e.g., cigarette and cosmetic packaging) and flexible packaging on paper, board, and film. This is especially true when only small areas are covered with aluminum where metallized paper or film has to be overprinted with standard colors in the areas where the design does not allow a metallic look.
Due to the fact that PVDA pigments were originally only offered as dispersions in various acetates, the main applications were gravure printing, followed by only a few screen applications (e.g., on compact disks, signs, etc.) This limitation completely excluded the flexo market, where suitable formulations are mainly based on alcohols and water. Since the beginning of 1999 new alcohol and water compatible dispersions are available that now open new possibilities within the wide-web and narrow-web flexo market where metallized paper or film and especially foil stamping were used to create brilliant metallic effects.
Typical formulations contain approximately 3-4% (gravure) or 6-7% (flexo) PVDA pigment (vs. approximately 15-20% conventional pigments), and have a pigment/binder-ratios ranging from 2:1 to 1:2, depending on the application.
Typical binders used include nitrocellulose, CAB, CAP, PVB and acrylics.
Coatings
|
| Click to enlarge |
In terms of metallic pigments, the decorative coating market is focused on pigments offering extraordinary lightness and flop effect.
In addition to the above mentioned requirements for applications based on PVDA-pigments, in the case of two-coat systems the clearcoat has to be adjusted to the colored basecoat. The clearcoat should initiate the dissolution of the basecoat just as far as absolutely necessary to achieve adhesion without disturbing the orientation of the flakes. Often a compromise between adhesion and effect has to be found.
Currently, PVDA pigments are used in coatings application such as bicycles, rims, glasses, watches, mobile phones, TVs, household utensils, sanitary facilities, and automotive interiors and accessories for cars such as door handles.
Due to the technical limitations already mentioned, typical formulations today are still based on acetates, glycols and ketones and typically contain 1–1.5% PVDA pigment (vs. approximately 3.5% conventional pigment in a low-solid basecoat) and approximately 4.3% binder (vs. approximately 19% in a low-solid basecoat).
The latest developments are in the OEM field to achieve mirror-like effects as well as metallic effects with an extremely deep flop. In this area, new waterborne formulations are currently under development.
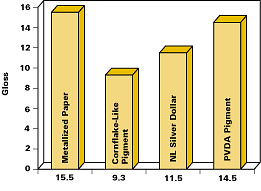
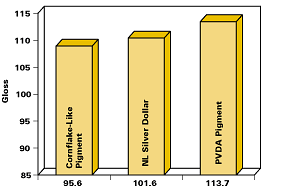
Figure 15 compares gloss values of PVDA pigments with those of conventional pigments measured on typical coating applications.
Conclusion
In this article we have discussed a special class of aluminum pigments which are manufactured according to a PVD-process. The manufacturing process of these PVDA pigments allows a defined and controlled adjustment of all relevant physical and chemical properties of the deposited metallic layer and provides a unique combination of excellent effects. •
- Highest brilliance of all metallic pigments •
- Best hiding power of all metallic pigments •
- Strongest flop effect of all metallic pigments •
- Most perfect nonleafing behavior of all metallic pigments •
- Most suitable for tinting •
- Perfect rub resistance •
- Excellent intercoat adhesion
These pigments open up a range of application possibilities meeting nearly all requirements of designer, stylist and formulators. Although these pigments have been on the market for more than 10 years, they still represent a niche product.
However, their increasing acceptance coupled with better understanding of the product and decreasing manufacturing costs will open up an interesting future for these products.
For more information on aluminum pigments, contact Eckart Werke GmbH & Co., Plant Guentersthal, D-91235 Velden, Germany; phone +49/9152.77514; fax +49/9152.77508; e-mail j.seubert@eckart.de.






Report Abusive Comment