Intumescence is generally accomplished with a minimum of three components: a source of mineral acid catalyst (typically ammonium polyphosphate, APP), a source of carbon (typically pentaerythritol or dipentaerythritol), and a blowing agent (typically melamine). When an intumescent coating is subjected to heat, a series of chemical reactions occurs: the ammonium polyphosphate decomposes to produce phosphoric acid; the phosphoric acid causes dehydration of the pentaerythritol or dipentaerythritol to produce a carbon char; the blowing agent decomposes, releasing non-flammable gases that cause the carbon char to foam, thus producing a meringue-like structure that is a highly effective insulator against heat.
The basic function of the coating binder is to bind together these intumescent ingredients, and to provide adhesion to the substrate, so that they are held in intimate contact, in order to perform their function when required to do so in a fire situation. Furthermore, it contributes to the formation of a uniform cellular foam structure since the molten binder helps trap the gases given off by the decomposing blowing agents, thus ensuring a controlled expansion of the char. It is important that the ingredients retain their functionality over a long period of time, so the binder also has to protect the often water-sensitive intumescent ingredients by providing the necessary resistance to water, UV light, abrasion, etc.
Eliokem resins have been widely used in solventborne intumescent coatings for many years. However, the contribution of the resin to the intumescence process is not well understood, and very little published data exists on the subject. With this in mind, we embarked upon a comprehensive study of the interaction of the main thermoplastic resins used for solventborne intumescent coatings with the principal intumescent ingredients, in order to gain a better understanding of the mechanisms involved. This would aid in developing new, improved binders for this application, in line with increasing demands from the industry.
The study was performed in collaboration with a French laboratory, CREPIM (Centre de Recherche et d'Etude sur les Procedes d' Ignifugation des Materiaux), that specializes in the fire protection of materials.
Experimental
Materials
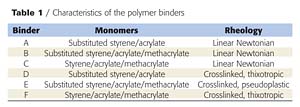
The nature and characteristics of the binders (supplied by Eliokem) used in this investigation are reported in Table 1. Ammonium polyphosphate (APP)/binder mixtures were processed using the following method. First, the binder is dissolved in xylene (laboratory reagent grade). Then, the APP (Hostaflam AP422 from Clariant) is dispersed into the solution, and the solvent is then evaporated at 60 °C until constant weight is attained. The APP/binder mixture is ground in liquid nitrogen using an ultra centrifugal mill (Retsch ZM100) to avoid degradation. APP is added to the binder at 5 wt % phosphorus content.
Intumescent coatings were prepared using APP as acid source, melamine as blowing agent and dipentaerythritol as carbon source, and have been applied onto the surface of a metallic support (10 x 10cm2). Thick films of about 1mm were obtained by deposition of 1100g/m2.
Thermogravimetric Analysis
Thermogravimetric analyses were carried out at 10°C/min under synthetic air (flow rate: 5 x 10-7 m3/s) using a Setaram MTB 10-8 microbalance. In each case, the sample (around 10 mg) in the form of powder was placed in an open vitreous silica pan. The precision of the temperature measurements was 1.5°C over the whole range of temperature.In order to determine whether a potential increase or decrease in the thermal stability of the polymer is related to the presence of the additive, the curves of weight differences between experimental and theoretical TGA curves were computed as follows:
-
Dw(T): curve of weight difference at the temperature T
(p)
Dw (T) = Wexp(T)-Wthe(T)
Wexp(T): experimental TGA curve of polymeric binder/APP,
Wthe(T): theoretical TGA curve computed by linear combination of the TGA curves of the binder and APP:
Wthe(T) = x Wpoly(T)+ (1-x) Wadd(T), with x = polymer weight fraction
Wpoly(T): TGA curve of the polymeric binder
Wadd(T): TGA curve of APP.
Dynamic Viscosity Measurement
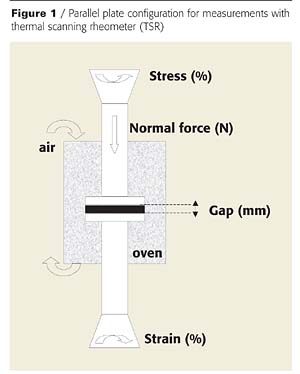
Rheological measurements were carried out using a Rheometric Scientific ARES-20A thermal scanning rheometer (TSR) in a parallel plate configuration (Figure 1). A dynamic viscometer measures elastic together with viscous behavior by determining the response to both steady rate and oscillatory shear. The TSR measurements are designed to monitor the changes in rheological properties with temperature and/or time.
Samples of dry coating (diameter = 25 mm, thickness = 1 mm) were positioned between the two plates (25 mm diameter) with a starting gap of around 1 mm. A constant normal force was systematically applied in order to obtain good adhesion between samples and plates, and to guarantee the validity of the results. First, the viscosity measurements were carried out varying the value of the stress, the normal force and the frequency in order to define the best operating conditions for determining the viscosity values over the complete temperature range (20 - 500°C). The best conditions are the following: strain value: 10%, frequency: 10 rad.s-1. These conditions enable the material to be preserved during the formation of intumescence. The tests were realized in the 180 - 400 °C range of temperature with a heating rate of 10 °C/min.
Fire Resistance
Temperature profiles were measured at the interface between the intumescent coating and a metallic plate for a sample exposed to a constant heat flux of 35 kW/m2 under the conditions of the cone calorimeter.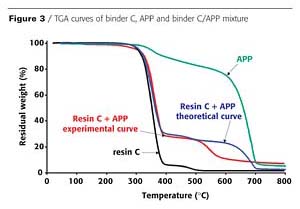
Results and Discussion
Thermal Degradation
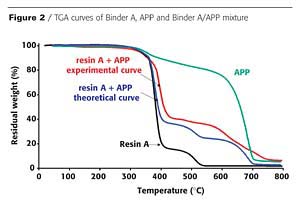
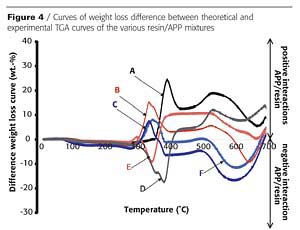
In an intumescent process, the first stage consists of a reaction of the acidic species released by the acid source with the carbonization agent with formation of a mixture of esters. However, it has been reported that the polymer can contribute to the intumescence process.3 The chemical reactivity of the resin with the APP is investigated here using thermogravimetric analysis (TGA). Figures 2 and 3 show the TGA curves of binder resins (A and C), of APP, and of the resin/APP mixture. The theoretical degradation curve of the mixture resin/APP is also represented.
The difference between the experimental and the theoretical TGA curves gives information on the reactivity of the binder resin with APP. When the experimental curve is above the theoretical one, the loss of weight is lower than expected, showing that the reactivity of the resin with APP leads to a thermal stabilization of the materials (i.e. synergism). If the experimental curve is below the theoretical one, the reactivity of the resin with APP leads to a thermal destabilization of the materials (i.e. antagonism).

Rheological Measurements
In order to understand why the expansion varies depending on the binder resin, the apparent viscosity of the binder resins and of the intumescent paints made from them, have been successively measured and are shown in Figures 5 and 6.
The dynamic viscosity of binder resins alone is measured versus temperature (Figure 5). Whatever the nature of the monomer, the viscosity is very low, and no charring is observed in the temperature range corresponding to the development of intumescence (between 350 °C and 400 °C). A slight increase in the apparent viscosity is observed when crosslinked resins are added to linear resins. The behaviors observed correspond to those of thermoplastic materials. No obvious correlation can be found between these viscosity measurements and the difference in the char development observed with these resins.

This correlation between the coating viscosity (in the range of temperature at which the intumescence starts) and the efficiency of the char formation is perhaps not surprising. If the viscosity is too low, the gases escape from the char layer and feed the flame. If the viscosity is sufficiently high, slow diffusion occurs with the consequent formation of an expanded structure.

Fire Protection
The temperature profiles measured using the cone calorimeter at the interface of the intumescent coating and the metallic substrate are reported in Figure 7. The shape of the temperature profiles is similar for all the coatings. The temperature increases rapidly during the first 400 seconds and then the temperature stabilizes and a plateau is achieved. The temperature at the plateau is slightly lower with the coating prepared with binder C (i.e. with the binder that shows no reactivity with APP). So, looking at the temperature profiles obtained with the Newtonian resins, no correlation can be observed between the reactivity with APP, the expansion rate and the thermal insulation given by the coating. However the difference of temperature at the plateau obtained with the different resins A, B and C is about 30 °C, which is within the experimental error.When the coatings are formulated with a blend of Newtonian and crosslinked resins, a significant improvement of the thermal insulation is observed. This is particularly evident with the binders A and B prepared with substituted styrene. The temperature at the plateau is decreased by about 100 °C.
The crosslinked resins have the same monomer compositions as the Newtonian resins to which they are blended but have a crosslinked structure. The effect of the crosslinked resins is a modification of the rheology of the binder and thus of the coating during the intumescence process. This was discussed prior. The better thermal insulation obtained by the introduction of the crosslinked resin can be attributed to a better control of the diffusion of gases during the intumescence and consequently to an optimization of the char development and structure.

Design of New Latex for Waterborne Intumescent Coatings
Current latexes used to prepare waterborne intumescent coatings for structural steel protection, are predominantly copolymers of vinyl acetate, which are effective in terms of intumescence but unfortunately show very poor durability and resistance to water. Their intumescent efficiency in terms of film thickness required for a given period of fire resistance is related to the relatively low melting temperature of vinyl acetate coplymers, which allows for a high level of expansion of the intumescent foam. The drawback of this is the ‘stickability', or the ability of the intumescent coating to adhere to the steel without slumping, cracking or falling off under fire conditions, and consequently in limited (shorter) fire-resistance times. The advantage of a styrenic/acrylic copolymer is the potential to enable the formulation of durable waterborne coatings, which is of particular interest when the steel sections are to be precoated in-shop (off-site application).
Taking into account the results obtained with the resins for solventborne intumescent coatings, an attempt was made to develop a polymer latex, which could be used as binder for improved waterborne intumescent coatings.
Thermal Degradation
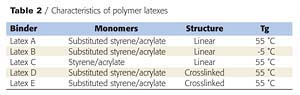
Three linear latexes and one crosslinked latex were prepared by emulsion polymerization with styrene, substituted styrene and acrylic monomers. The latex characteristics are given in Table 2 (see page 70). The latexes A and D have respectively the same composition and characteristics as solventborne resin A and resin D. Latex B was prepared with the same monomers as latex A but with a much lower ratio of substituted styrene. As a consequence, its glass transition temperature (Tg) is lower than that of latex A. Latex C was prepared with styrene with the same Tg as latex A. Intumescent coatings were prepared from linear latexes A, B and C and from an 80/20 blend of linear polymer latexes and crosslinked latex.
Blends of APP and polymer latex were prepared and dried, and then the potential increase or decrease in the thermal stability of the polymer related to the presence of the APP was determined by the weight difference curves between experimental and theoretical TGA curves (Figure 8), as described previously with resins. Here too, better thermal stability was found with polymers prepared with substituted styrene but to a lesser extent than with resins. The crosslinked polymer shows the highest stability.

Fire Protection
Waterborne intumescent coatings were prepared with the various latexes and tested for fire performance as was previously described for resins. Temperature profiles obtained at the interface between the intumescent coatings and the metallic substrate were measured using a cone calorimeter with a heat flux of 35 Kw/m2.Results are shown in Figure 9. As for resins, the best thermal insulation was obtained with latex A containing the highest level of substituted styrene. It can also be observed that whatever the linear latex, the addition of the crosslinked latex in the coating formula induces a significant improvement of the thermal insulation.
The results obtained with polymer latexes for waterborne intumescent coatings are comparable to those obtained with the resins for solventborne intumescent coatings.
Further testing is in progress to assess the fire protection over longer time periods and at higher heat flux. Furthermore, furnace tests are being conducted using equipment currently used to assess the performance of these types of coatings, according to standards recognized by intumescent coatings' manufacturers and users.

Water Resistance
The resistance to water or humidity of the acrylic intumescent coatings was assessed by water immersion and wet scrub tests. Results were compared with those obtained with PVA copolymer latex paint of the same composition.Significant early water resistance improvement of intumescent coating can be obtained with the experimental latexes. Figure 10 shows the results for an intumescent coating prepared with latex E, applied to a steel substrate and dried for 36 hours, after 24 hours water immersion, compared to a coating based on PVA copolymer of the same formulation. It can be seen that the intumescent coating formulated with PVA latex has swollen and detached from the substrate whereas the coating prepared with experimental latex E is fully intact, and adherent.
Latex intumescent coatings were applied to a Leneta chart at 300 µm wet and allowed to dry for 36 hours at room temperature before being tested for wet scrub resistance. Results are shown in Figure 11. The intumescent coating prepared with PVA latex was completely removed after only 200 cycles. The intumescent coating prepared with the experimental latex E resisted 7000 cycles before the substrate became visible.

Conclusions
Results from this work substantiate the importance of both the chemical and physical properties of the coatings on the intumescent efficiency as already found in previous study.6 The importance of the chemical nature of the polymer binder is highlighted by the reactivity with ammonium polyphosphate, which is better with substituted styrene than with styrene. The improvement of the thermal insulation, afforded by the char during a fire test, when a crosslinked resin is present in the binder, is clear evidence of the crucial role of the rheological properties of the binder under the test conditions, particularly in the range of temperature at which the char formation starts. Optimum fire protection is obtained when the binder is composed of a mixture of linear and crosslinked polymer and contains substituted styrene as co-monomer.
Waterborne latex binders for intumescent coatings have been developed based on the conclusions derived from the study of solventborne resins. These duplicate the behavior of their solventborne counterparts in terms of fire resistance, and permit the formulation of intumescent coatings with improved water resistance compared to the latexes currently used in this application.
Acknowledgements
The authors wish to express their thanks to Drs. S. Duquesne and C. Jama of the Ecole Nationale Superieure de Chimie de Lille, and Prof. R. Delobel of CREPIM, for their significant contribution to this investigation.
This paper was Presented at the International Waterborne, High-Solids, and Powder Coatings Symposium, February 18-20, 2004, New Orleans.
References
1 Vandersall, HL. Intumescent Coating Systems, Their Development and Chemistry. Journal of Fire & Flammability, 1971; 2(April): 97-140.
2 Rhys J.A. Intumescent Coatings and Their Uses. Fire and Materials, 1980; 4(3): 154-156.
3 Le Bras, M.; and Bourbigot, S. Fire-Retardant Plastics. International Plastic Flammability Handbook - Fundamentals. New York: Hanser, 2003. Under publication.
4 Duquesne, S.; Magnet, S.; Jama, C.; and Delobel, R. Intumescent Paints: Fire Protective Coatings for Metallical Substrates, European Materials Research Society Spring Meeting, Strasbourg, June 10-13, 2003.
5 Grassie, N.; and Scott, G. editors. Polymer Degradation and Stabilization. Cambridge: Cambridge University Press, 1985.
6 Duquesne, S.; Delobel, R.; Le Bras, M.; and Camino, G. A Comparative Study of the Mechanism of Action of Ammonium Polyphosphate and Expandable Graphite in Polyurethane. Polymer Degradation and Stability 2002; 77: 333-344.


Report Abusive Comment