
Polyurethane dispersions are aqueous-based systems consisting of PU particles ranging from 50 to 500 nm in diameter, frequently with some additional organic solvent present. The PU particles typically comprise from 30 to 45 weight percent of the total dispersion (% solids). The Dow Chemical Company produces PUDs using a patented, continuous mechanical dispersion process DisPURsa**.1,2 This process allows the production of solvent-free PUDs with high solids levels (>50%). Solvent-free PUDs permit lower production costs as well as facilitating the ability to meet environmental regulatory pressures. In addition, these PUDs can be prepared from a variety of polyols (i.e. polyester, polyether, etc.) and with either aliphatic or aromatic isocyanates. The latitude that is provided by this process allows the production of a wide range of materials to meet a broad range of cost/performance targets.
Although PUDs have been commercially available for many years, their use has been limited due to their higher cost. The continuous dispersion process can reduce the overall cost of the PUD either through the use of lower-cost raw materials or a lower process cost. However, if the usage of PUDs is not established for a given application, it will require the development of additional formulation expertise to take full advantage of the benefits of the PU polymer. It is important to note that PUDs do not behave in a similar manner as solvent-based PU systems or even other aqueous SB latex materials. The objective of this paper is to establish an understanding of PUDs and to aid in the formulation science particularly in coatings applications.

Experimental
The PUDs used in this study were all produced by The Dow Chemical Company. General properties of these PUDs are shown in Table 1. Films were made with these materials either by pouring the PUD into a mold or by using a drawdown method. The films were allowed to dry overnight at ambient conditions and then cured in the oven at 120 °C for 20 minutes.The mechanical properties (tensile strength and elongation) of the films were measured using either ASTM D 1708 or DIN 53504 testing methods. Tear resistance and water permeability of the films were measured in accordance with ASTM D 1004 and ASTM E 96, respectively.
Resistance to water and other solvents was done by either of two methods. In the first method, the film was immersed in water or solvent for a designated time. This was followed by the measurement of the amount of solvent that was absorbed during this time. The second method was a spot test in accordance to ISO 2812. Ratings on this test went from 0 (Film destroyed) to 5 (No visible change).
Tuffbind was measured on carpet backings coated with compounded PUD. Testing was done in accordance to ASTM D 1335.
Results and Discussion
Crosslinking
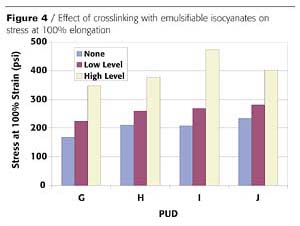
Polyurethane dispersions contain polymers in which the molecular chains are linear or lightly branched. Due to the polar nature of these chains, an extensive amount of hydrogen bonding may be present in the polymer. Thus, under certain conditions, these materials may be sensitive to water or other solvents. In addition, performance at higher temperatures may prohibit their use unless formulation modifications are made. Many of these limitations can be remedied through the use of crosslinking agents. Typical crosslinking agents that can be used with PUD include organofunctional silanes and emulsifiable isocyanates. If the PUD contains carboxylate groups, crosslinking with carbodiimide or polyaziridine agents is also possible.3
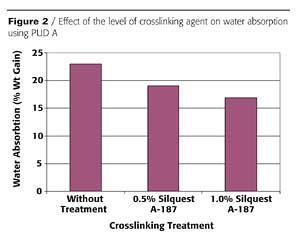
Solvent resistance can also be improved using organofunctional silanes. Figure 3 shows the effect of organofunctional silane crosslinking on solvent resistance of PUD A as measured by a spot test. This figure demonstrates the effectiveness of crosslinking as the solvent resistance to acetone, butyl acetate and isopropanol (IPA)/water is greatly improved when using silane additives. In these spot tests, the level of the organofunctional silane had only a slight effect on the solvent resistance rating.

Although not required, the Dow process allows for the introduction of carboxylate groups in the polymer structure. When this is done, the options for crosslinking increase to include the opportunity to use polyaziridine or carbodiimide crosslinking agents. An example of these treatments is shown in Figure 5. This figure shows the percentage weight gain for four different polymer films, with and without crosslinking treatment, that have been immersed in a IPA/water (70%/30%) solution for one week. PUD K has no carboxylate groups in its structure, whereas, PUD L, M and N have carboxylate groups and are arranged in order of increasing carboxylate content. As can be seen from this figure, the solvent resistance decreases as the carboxylate content increases with the untreated polymer. However, with the addition of crosslinking agents, the solvent resistance shows large improvements that increase with increasing carboxylate content.

Plasticizers
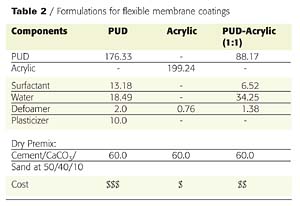
Plasticizers can be used with PUDs to increase formulation flexibility and in many cases lower cost. Plasticizers will reduce the glass transition temperature of the dried film, reducing tensile strength, hardness and modulus while increasing elongation, flexibility, particle coalescence and processability. An example of how properties change as plasticizer is added to the system is shown in Figure 6. In this case, the tensile strength of the polymer film decreases as plasticizer is added. Comparable results are obtained for the two plasticizers shown. In addition, because a compatible plasticizer increases the binder volume, higher pigment/binder ratios can be acceptable.
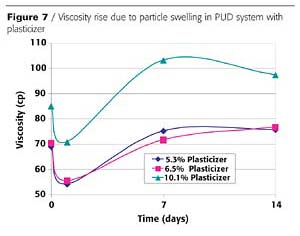
Compatibility should be tested with each plasticizer. In general, plasticizers are not soluble in water, however, upon mixing they become a dispersed phase by the emulsifying system within the PUD and end up partitioning into the PUD polymer particles. As a plasticizer is added to a waterborne PUD formulation, the polymer particles swell, raising the viscosity of the dispersion. Thus, viscosity rise can be used as a measure of plasticizer compatibility. An example of this swelling phenomenon is shown in Figure 7.

Fillers
Inorganic fillers, also known as extender pigments, are particles that add bulk, reduce cost, provide opacity and color, control rheology and modify specific properties of a coating, adhesive, sealant or elastomer. Typical fillers are calcium carbonate, talc, clays, silicas, titanium dioxide and carbon black.
There is a limit to how much filler can be added while maintaining acceptable properties. Mechanical properties of filled systems are heavily dependent on critical pigment volume concentration (CPVC), which is affected by the packing and size ratio of the filler. Physical properties of filled systems that are above their CPVC tend to deteriorate. However, it is possible to design a polymer structure that has better filler compatibility to better maintain mechanical properties as filler level is increased. Figure 8 shows an example of how the properties of a material change as filler loading level increases. The property that is illustrated here is that of tuffbind, a property that measures the strength of a coating used for carpet applications. In this case, PUD D has superior performance in both strength and strength retention as filler level increases.
Systems with Other Waterborne Polymers
In addition to inorganic fillers, properties can also be altered through the use of organic fillers in the form of acrylic, styrenic and vinyl acetate emulsion polymers. As with inorganic fillers, these emulsion polymers will also allow for decreases in the total formulation costs, thus providing the benefits of a polyurethane system with reduced cost.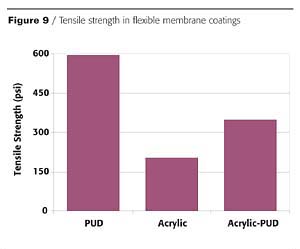



Summary and Conclusions
Dow's continuous dispersion process allows the production of economic, viable PUDs possible for numerous coating applications. These PUDs form the basis of an initial product offering designed to meet many coatings customers' needs with PUDs. To further expand the application of these materials, various formulation aids can be employed. These formulation aids include crosslinking agents, plasticizers, fillers and other waterborne polymers. By utilizing these and other techniques, PUDs can be successfully applied to multiple coating applications in a cost-effective manner.Acknowledgements
The authors would like to acknowledge Yves Gentil and Alain Lejeune from Crompton Corporation-OSI Specialties, for their contribution in the silane crosslinking work. Thanks are also given to Ute Bertheas from Dow UES, for help with acrylic dispersions, and to Antoine Storione, Bob Kuklies and Dr. Hans Kaul for their lab support.
References
1 Skaggs, K.; Tabor, R.; Louks, P.; Willkomm, W. United States Patent 6,087,440; (2000).
2 Erdem, B.; Robinson, D. Polyurethane Expo 2002, p. 267, (2002).
3 Coogan, R.G. "Post-crosslinking of Waterborne Urethanes'' Progress in Organic Coatings; 32; 51-63; (1997).
This paper was presented at Polyurethanes Expo 2003, Oct. 1-3, 2003, Orlando.


Report Abusive Comment