Until the early 1970s, ink chemistry was based entirely on organic solvents. The coatings industry in general relied on low-solids solvent systems (~10% solids). Such a high solvent content resulted in the release of a large amount of VOCs. During this period, chemists depended heavily on the molecular weight aspect of the polymeric materials to fine-tune the performance characteristics of the system. Naturally, high-molecular-weight polymers demanded high solvent/solid ratios.
When the necessity of switching over from solvent systems became imminent, a two-pronged approach was adopted. The first one was the obvious choice of using water as solvent, and accommodating high-solid content. The second one, which was more imaginative, centered on manipulating the molecular weight problem of the polymer. In this approach, monomers and low-molecular-weight polymers (oligomers) were substituted for the high-molecular-weight ones. What was needed was a mechanism to initiate the in-situ polymerization of these species that would cure the coating. This step could be carried out by a variety of methods. The simplest means of using heat (thermal curing) did not become popular since relatively high temperatures were necessary to trigger the polymerization process, even in the presence of chemical initiators, which could be detrimental to many substrates.
It is against this backdrop that the potential of radiation and photochemistry to polymerize monomers and oligomers was thought to be worthy of consideration. High-energy radiation and photons have a reputation for being clean reagents, in that they do not leave any of their fragments in the reaction medium. These special reagents, though expensive, were unique in their effect and occupied a special position in the arena of chemical reactions. This article pertains to the special chemistry induced by radiation and photons, respectively, in the context of inks that have relevance to paints and coatings also.
The term “radiation” embraces electromagnetic radiation such as visible light, ultraviolet (UV) light, X-rays and g rays, as well as atomic radiation such as electrons, neutrons and g-rays. Among them, the first two are of low energy and others are considered as high energy radiation. The chemistry induced by high energy radiation, whether electromagnetic or atomic, is somewhat similar.
Electron-Beam Curing
Electron-beam-cured ink is gaining prominence as an environmental friendly alternative. Some of the projected improvements in the product and process include: highest gloss, highest resistance to mechanical and chemical factors, elimination of solvents/nearly 100% solids, fastest line speeds, instantaneous curing, consistency in batches and low temperatures.2These positive gains in performance characteristics are derived from the ability of electrons to form crosslinked polymeric films and to penetrate the surface. Added to this is the decreasing trend in the cost of the irradiation facility and improvement in the performance of constituent chemicals. A favored approach to radiation technology is reflected in the popularity of RadTech conferences. The electron was discovered by J.J. Thomson in 1897 while working in the Cavendish laboratory in United Kingdom. His experiments on cathode rays led to the revolutionary concept of the existence of subatomic particles. The modern understanding of electrons considers it to be a member of the class of atomic particles called leptons, the other important particle being quarks. Nuclear particles such as neutrons and protons also are made up of these. In an atom, the negatively charged electrons are positioned outside the nucleus in orbitals of increasing energies. Their number is equal to the number of positively charged protons. Chemists regard electrons very highly, since their participation is responsible for controlling the chemical reactions. Electricity is nothing but the movement of electrons, and each particle carries a charge of 1.602 x 10-19 coulombs.
High-energy electron beams are produced by accelerating electrons in a particle accelerator. Energetic electron beams thus produced can cause chemical changes in the ink coating. The electrons that are generally obtained by heating a metallic cathode in a vacuum chamber may be accelerated by two techniques. In the first one, the accelerating force, which is an electric field is derived from an electrostatic potential. In the second one, the required electric field is provided by the electric field component of electromagnetic radiation3,4 that usually lies in the radio frequency or microwave region. The Van de Graff generator and Linear Accelerator (linac) respectively work on these principles. These accelerators can impart electron energies well above 1 MeV (million electron volt). But in the actual ink scenario, much lower electron energies are used, typically in the range of 125–150 keV (kilo electron volt). Such an energy range may be attained by a single gap accelerator technology,5 where the beam current can be several milliamperes.
Radiation chemistry is concerned with the interactions of energetic charged particles and high-energy photons with matter. The radiation chemistry brought out by high-energy electrons is very similar to that induced by photon sources such as X-rays and g-rays. Since these sources cause ionization of the medium in which they traverse, they are also known as ionizing radiation. Conversely, UV and visible light that do not cause ionization are called nonionizing radiation.
When electrons collide with matter and interact, two types of processes take place: scattering and nuclear capture. Scattering is the interaction in which the particle retains its nature or maintains identity. It can be either elastic type or non-elastic type. In the elastic type, electrons do not lose energy irrespective of the scattering angle. In the non-elastic type, electrons lose energy. The energy loss can result in atomic displacement, excitation or ionization. Thus only the inelastic interactions are of any chemical consequence. At relatively high electron energies and large scattering angles, an energy loss mechanism referred to as Bremsstrahlung occurs, which is the light emission due to the deceleration of electrons.
When electrons interact with matter, they collide with the electrons in the atom and transfer some of their energy to them. These electrons can be excited to higher energy levels and at appropriate electron beam energies, the atom can lose that electron producing a positive ion. These ejected electrons are known as secondary electrons. These low-energy electrons will dissipate energy over a small volume in the medium that results in little clusters of ionizations and excitations. These spherical clusters are called spurs and have a diameter of ~2 nm (nanometer).
Some of these secondary electrons may be captured by a positive ion and produce a highly excited state that can dissociate into other products. A fraction of these electrons might be simply trapped by the molecules and solvated. In water they are referred to as hydrated electrons.
Inks used in EB curing make use of the polymerizing capability of acrylic monomers and oligomers. Acrylic chemistry has a special significance in modern day inks.6 The structure of the simplest acrylic compound, acrylic acid, is shown in the equation below.
CH2=CH-COOH
The double bond in the acrylic moiety opens up during interaction with electrons (initiation) and forms a free radical that acts on other monomers forming a chain (propagation) leading to high-molecular-weight polymers. It may be noted that in radiation induced polymerization, no external initiator is needed since radiation itself generates free radicals with the result that no initiating species will be left in the coating unlike in a UV cured coating. However, radical scavengers like oxygen should be eliminated from the molecular neighborhood to prevent the removal of the crucial initiating alkyl radicals as alkyl hydroperoxide radicals. Otherwise, an induction period will be induced in the polymerization reaction and longer irradiation time (higher doses) will be required for the sustenance of polymerization. This would affect the press speed. Purging the reaction environment with an inert gas like nitrogen would eliminate this problem. Removal of oxygen is also advantageous since radiation could convert oxygen into hazardous triatomic ozone. Moreover, radiation has the effect of producing crosslinks that can add to the mechanical properties of the coating. This is in addition to the cross linking effect caused by the polymerization of multifunctional monomers and oligomers. However, the degrading effect of radiation on the other constituents of the ink, especially the color-imparting pigments, should be considered.
It may be interesting to introduce the effect of radiation on polymers themselves, even though such a continuous irradiation of ink coating by electrons does not happen. With radiation, radicals will be generated on the polymer chain. For example, in the case of polyethylene, C-H and C-C bond scission occurs. The former type of radicals can respond by eliminating a hydrogen atom from a neighboring CH2 group forming a double bond. Two of them may combine, forming a crosslink that results in a high-molecular-weight polymer. On the other hand, radicals generated from the C-C bond breaking undergo elimination reactions, producing low-molecular-weight fractions.
A variety of acrylic monomers are available for EB curing that range from simple acrylates such as 2-phenoxyethyl acrylate and isooctyl acrylate7 to prepolymers like bisphenol A epoxy acrylate and polyester/polyether acrylates.
Radiation-induced curing technology is met with some amount of skepticism by the general public since it is linked with exposure to radiation and the fear of radioactivity. But, electron beam in this energy range does not induce any radioactivity to the product. However, caution must be exercised with these sources by protecting them with appropriate shields that absorb stray particles and harmful X-rays emanating as secondary radiation.
It is natural that the industries concerned very often compare the EB technology with UV technology since they compete with each other.2,8 In spite of the downside of the high investment involved in EB technology, it is superior to UV technology in being more acceptable, as it does not leave any initiator fragments. Furthermore, its penetration is dependent only on the density of the coating and not restricted by specific absorption ranges as is the case with UV that may be masked by certain pigments. These factors make them more acceptable in food packaging where FDA regulations exist.
Ultraviolet Radiation Curing
Ancients worshipped the sun intuitively, probably unaware of the immense potential it embodies in shaping the destiny of humans. Life as we know on this planet sustains with the energy derived from sunlight. Plants absorb solar radiation, converting it into carbohydrate by combining carbon dioxide and water through photosynthesis. This is the first link in the great chain involving animals that consume plants, both of them contributing to fossil fuel on their decay.The nexus between ink and light originates from the effect of light energy on ink coatings. Primarily, the lightfastness of colored prints depends on the interaction of the UV components of light with the pigments. Inks with superior light resistance were once branded as UV inks. Now, the term “UV ink” refers to that ink that is cured by ultraviolet light.
UV inks came to light with the need to circumvent the VOC problem, since the inks provided a solventless, nearly 100%-solids system. These inks had their own special appeal. They improved the color density since all the coated material remained on the substrate after curing. They imparted superior image quality and print quality due to reduced color bleed and improved dot gain. They dramatically enhanced the printing efficiency in terms of print speed and uniformity. Other positive attributes are improved rub resistance, chemical resistance and superior gloss. Still, properties such as lightfastness and opacity could be retained by choosing the appropriate pigments.
Moreover, these inks are supplied as press ready, minimizing printer setup time. Properties such as uniform consistency also contribute to maintaining uniformity in prints, thus eliminating subjective variation since the variability from operator to operator and press run to press run is minimal. These are aside from the positive gains in the efficiency of printing machinery itself. UV inks do not dry in air; hence, they do not tend to plug the cells of the anilox rolls in flexography. Since the inks are devoid of solvent, there is no need to clean them between press runs; cell can even be left overnight or even through the weekend without cleaning.
UV inks and UV curing technology have been successfully applied in many areas: A major sector is the food packaging industry. In the United Kingdom, about 75% of all litho cartons are printed with UV ink. In Denmark, bank notes9 are printed by this technology. More esoteric applications like printing on CD, and, of late, printing on DVD are also in vogue. A variety of substrates such as metal, paper, glass, wood and plastics have been printed with UV ink. The most popular printing techniques that use UV ink are flexography and screen printing.
A typical UV ink consists of monomer and/or oligomer, pigments, photoinitiators, and other additives.10 The ink coating applied by the appropriate method is polymerized or cured by exposure to UV rays.
UV curing methodology shares many aspects in common with EB technology. The central process in UV curing is the UV light induced polymerization of monomers and oligomers through the mediation of photoinitiators. It is in this context that the importance of photochemistry becomes significant to ink chemists.
Photochemistry refers to the chemical consequences of light absorption by molecules. More specifically, it is concerned with studying a type of chemical reaction that is dependent on the action of visible or UV light. Photochemists consider light a reagent, not just a catalyst.
Many natural and synthetic processes such as photosynthesis, photography, and photopolymerization are examples of photochemistry in action. In medicine, the photodynamic therapy is a course of treatment for benign and malignant growth of tissue in which a photosensitizer medicine is administered intravenously and activated with laser light. Contributions in the last century have developed photochemistry into a mature science for which it has heavily drawn from other branches like spectroscopy and quantum mechanics.
Photochemical reactions spring from a state of the molecules called excited states. This refers to the excitation of electrons within the molecule to higher energy positions. The light energy absorbed by the molecules results in the creation of excited states. Regular chemical reactions are said to proceed through ground state mechanisms. Light absorption also helps in crossing the activation energy barriers associated with chemical reactions.
Photochemical processes are of primary concern to the UV curing process. Since chemical reactions are about bond breaking and bond making, let us see what happens when a bond breaks. When a covalent bond connecting two atoms, A and B, breaks, the cleavage can take place either by sharing the bond electrons equally or unequally. In the first case, free radicals are formed and in the latter case ions are produced. In other words, A:B (A-B) dissociates either as A. and B. (homolytic cleavage) or as A+ and B-/A- and B+ (heterolytic cleavage), depending on the electronegativities of A and B. How stable these species are depends on their structural and microenvironmental factors. In photochemical processes, cleavage into radicals is very common. Other common photochemical reactions are photoionization (e.g., acetylene at 110 nm), two-bond dissociation (e.g., diazirene at 313 nm to singlet methylene radiacal), isomerization (e.g., trans-stilbene to cis-stilbene), rearrangement (e.g., 1,3-butadiene to cyclobutene), hydrogen atom abstraction (e.g., photoreduction of benzophenone by toluene), and photoaddition (e.g., cyclodimerization of 2-cyclohexanone).
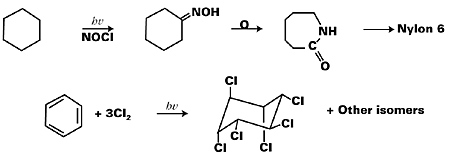
The UV curing process is considered to be one of the fast growing applications of industrial photochemistry. Among other industrial applications, the conversion of cyclohexane into Nylon 6 by photoreaction with nitrosyl chloride, transformation of 7-dehydrocholesterol into vitamin D3, synthesis of gammexane (an insecticide and a rodenticide) by the chlorination of benzene, and synthesis of surfactants by the photosulfochlorination of hydrocarbons are significant. Figure 1 represents some of these reaction schemes.
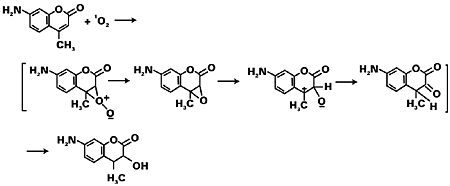

The last step repeats itself by adding to other monomers forming the polymer. The growing radical chains terminate either by the joining of two chains (combination) or by abstracting an atom from one of them (disproportionation).
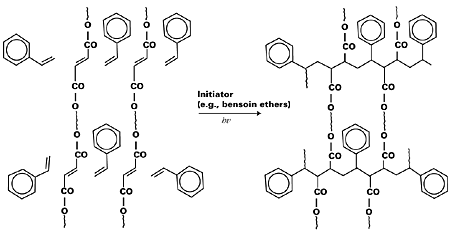
Cationic polymerization methods compete with radical polymerization in UV curing technology. Cationic photoinitiators are effective in polymerizing cycloaliphatic and aliphatic epoxides. They are usually Bronsted acids. Commercial cationic photoinitiators are aryl sulfonium hexa-fluorometallic salts. But free radical initiators are more commonly used since they provide faster curing. The printing speed in UV curing may be manipulated by the efficient choice of photoinitiators and their concentrations. Other parameters include the power of UV lamps, the nature of substrates, and the chemistry of resin components. At times, photosensitization reaction can be adopted to suit the requirement of initiators that do not absorb light directly. A case in point is the photoinitiator 2-methyl-1-[4-(methythio)phenyl]-2-morpholinopropanone-1 that is sensitized by the triplet sensitizer isopropylthioxanthone.13
Oxygen should be avoided in the neighborhood of photopolymerization. Oxygen retards the polymerization reaction by removing radicals forming peroxy compounds on one hand, and quenches triplet excited state of photoinitiators forming singlet oxygen. In the ground state, oxygen is an exceptional triplet designated by the spectroscopic state 3Greek sigmag- . This state quenches the other triplet excited states producing the two singlet states designated as 1Greek deltag and 1Greek sigmag+. While oxygen is harmful due to its depletive action on the excited state, the reactivity of the singlet oxygen formed from is itself another concern since they can act on many organic compounds. Oxygen inhibition problems may be tackled by purging the reaction medium and the immediate vicinity with an inert gas like nitrogen or argon. A molecular approach of incorporating a singlet oxygen scavenger such as tertiary amines covalently bonded to the oligomer backbone has also been attempted. Another negative aspect of oxygen in the vicinity is the possibility of producing obnoxious ozone by the action of UV light.
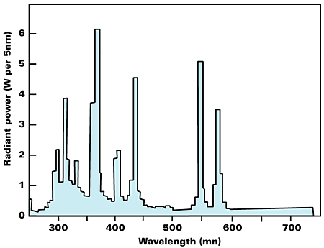
Flash lamps find applications for studying fast photochemical processes that needs high intensity, short duration flashes. Xenon flash lamps use gas discharge technique at a moderate pressure of about 6–20 cm, the energy for the flash being derived from a bank of condensers. Finally lasers are also used increasingly in advanced photochemical studies since they offer a convenient, highly collimated, monochromatic, and coherent beam of light.
UV curing is carried out by moving the product by conveyer under the UV lamp for 1–4 seconds of exposure. A lamp output of ~60–160 W/cm would suffice the intensity requirement. The UV dose needed is a function of the color of the ink since the pigments may absorb the UV component to different extents. Thus green and black inks need more UV dose than other colors. Also, the curing UV dose is dependent on the print thickness: the higher the thickness, the higher the dose needed.
An inevitable consequence of UV curing is the heat generated during UV irradiation. This can have negative effects on the coating. In sensitive cases such as printing on disposable cigarette lamps, the UV flash curing method14 can be adopted. Usually, one or two flashes from high-intensity flash lamps with a flash duration of 5–10 milliseconds cure the ink to a point to which it can be overprinted. The capacitors recharge in less than a second, and the total operating time is about 1 second per flash. Due to the abundance in the radical concentration produced in view of the high light intensity discharged by the flash lamp, oxygen quenching is not a problem in this case. This technique is projected as being highly suitable for multicolor printing/overcoating. Also, ozone formation is prevented. But the inks for this purpose should be specially designed to match with the high light flux of the flash.
The measurement of light intensities is necessary for controlling the absorbed dose and the photochemistry, both in basic and applied works. This is generally done by the method called actinometry, which is parallel to dosimetry in radiation chemistry. Absolute light intensities are determined using a thermopile galvanometer. A radiometer can also do the same job where thermistors replace thermocouples. Intensities of flash lamps and lasers can be accurately measured by a light calorimeter. But chemical actinometers are more commonly used in the UV region, the most important one being the ferrioxalate actinometer in which light absorption converts Fe(III) into Fe(II), and the Fe(II) is estimated by a colorimetric method. Phototubes calibrated against a thermopile galvanometer system can also be used to measure the absolute light intensity of monochromatic light.
Great advances in photochemistry have been made by the technique of flash photolysis since it brought to light the early events in the photochemical processes subsequent to light absorption that occurs in femtosecond (10-15S) time scales. Flash photolysis is to photochemistry what pulse radiolysis is to radiation chemistry. In flash photolysis, an ultrafast light pulse generated from a flash lamp or a laser intercepts the sample that produces transient species, which can be interrogated by another light pulse to analyze the transients. Frontiers of photochemistry are exploring attosecond (10-18S) time scale events with pulsed lasers.
UV curing technology has its own drawbacks. Stringent safety regulations should be followed since the UV light is extremely harmful to eyes and skin. The monomers and oligomers are toxic. Most of the initiators cause a bad odor aside from other toxic effects to skin. UV inks are more expensive than other inks. But, it is going to be an unquestionable alternative for many specific applications where other printing technologies fail to be effective. In that respect, its only competitor is the electron beam technology.



Report Abusive Comment