This problem can be countered through a range of hydrolytically stable polyester polyols developed by Uniqema, based on dimerized fatty acids.
Dimer Acids
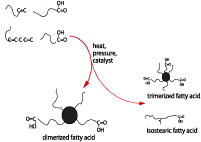
Natural oils and fats have for years provided polyurethane chemists with a variety of building blocks, such as glycerin and castor oil. Less known is the use of a fatty acid derivative, the so-called dimerized fatty acids, for polyurethane chemistry. These dimerized acids are obtained by the conversion of unsaturated fatty acids (from sources like soybean oil or tall oil) by a combination of pressure, temperature and catalysis. This process generates a mixture of products, the most important being dimerized fatty acid. Others are trimerized fatty acid and isostearic acid. Figure 1 gives an overview of the dimerization process.Starting from the C18 acids that nature typically provides, the dimer acid is a species with 36 carbon atoms, making it the longest dioic acid available. This hydrocarbon makes dimer acid and polymers in which it is included extremely hydrophobic. The combination of the hydrocarbon character and the non-crystallinity provides flexibility, even at very low temperatures, and lubricity.
Dimerized fatty acids have found application in such areas as polyamide epoxy curatives, polyester coatings, and solvent- and waterborne systems. In these applications, the value of the dimerized fatty acid is related to the features mentioned: flexibility and impact strength, wetting and flowability, and hydrophobicity and hydrolytic resistance.
Dimer Technology in Polyurethanes
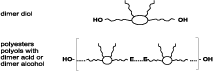
The dimer acids provide polyester and epoxy coatings with a range of favorable features, such as flexibility and hydrolytic resistance. Other coating systems could benefit from the introduction of dimer acids as well. Conversion of dimerized fatty acid to the corresponding diol, or by building dimerized fatty acid into hydroxy-terminated polyesters, makes it available for incorporation in polyurethane (see Figure 2).When comparing dimerate polyols to adipate polyesters, polycaprolactone polyols, and polyether polyols, it becomes clear that they form a category of their own. Compared to the adipates, much lower moisture absorption, a lower hydrolysis rate and a greater flexibility can be expected. Compared to polyethers like polyethylene glycol (PEG), polypropylene glycol (PPG), or polytetramethylene glycol (PTMEG), the absence of ether linkages will make dimer-based polyurethanes much more resistant to degradation by radical-type attack, such as heat, oxidation or ultraviolet radiation. The combination of stability against both hydrolysis and radical-type attack is unique, and highly relevant for applications including heavy-duty coatings and adhesives, and automotive elastomers. Additionally, it can be expected that dimer technology will bring low-temperature flexibility, flowability and affinity for low-energy surfaces; no monomer comes closer to a polyolefin than dimer acid.
A range of dimer-based polyester polyols is commercially available and has recently been tested in polyurethane elastomers and dispersions, in order to substantiate the above-mentioned assumptions.
Basic Properties
Initially, cured materials were tested by way of the preparation of polyurethane elastomers based on 1-part polyol, 2-parts butane diol chain extender and 3-parts pure MDI. Testing the dimerized fatty acid-based polyester polyols in polyurethane dispersion followed this study. Unless mentioned otherwise, the polyols had a molecular weight of 2000 g/mol.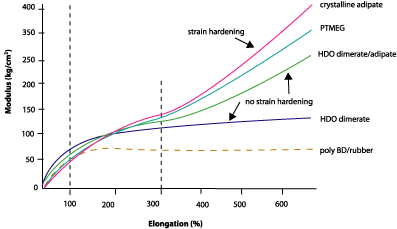
Water Uptake and Hydrolysis
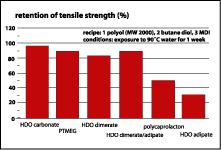
As a start, the expected hydrolytic stability of the dimer-containing PU elastomers was tested. Figure 3 demonstrates the performance of various polyol technologies after exposure of their polyurethanes to 90 deg C water for a week. It is obvious that the polyurethanes based on hexane diol adipate and polycaprolactone have undergone substantial hydrolysis. Hexane diol dimerate and a hexane diol ester of a mixture of dimer and adipic acids hold out very well. In fact, they are similar to PTMEG and polycarbonate diol. The explanation is in figure 4 -- the very low solubility of water in dimer-based systems very effectively reduces actual hydrolysis.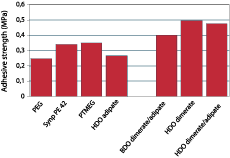
Mechanical Properties
The mechanical properties of dimer-based urethanes on elastomers made by 1-part polyol, 2-parts chain extender (butane diol) and 3-parts MDI show that the dimer technology offers some unique benefits. Figure 5 shows the mechanical properties of various PU elastomers as modulus vs. elongation. This graph shows that the modulus at 100% is comparable for all polyester and polyether polyols; the dimerates perform just as well as any other polyol. At 300% elongation, the picture is slightly different. For PTMEG and adipate, the modulus at 300% is double that of 100%. This can be explained by crystallization of amorphous polyol chains when oriented by the applied strain. The dimerate soft segment, on the other hand, is unable to crystallize because of the branched dimer structure. As a result, the modulus at 300% is only slightly higher than the modulus at 100%, although the increase depends on the structure of the polyol.In other words, the dimer-based polyurethane behaves more like a rubber. Any deformation is in principle reversible since no strain hardening takes place. The inability to crystallize when stretched (or the inability to strain harden) is also reflected in a relatively low strength when pushing the material to the limit of destruction. However, dimerate polyols produce a PU with similar elongation to any other technology, showing unimpaired untangling of soft segment.
Adhesion of Dimer-Based Polyurethanes to Various Substrates
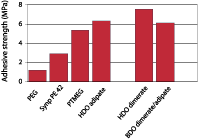
Dimerized fatty acids are expected to have a high affinity for low-energy surfaces, since the long alkane chains show a structure similar to polyethylene. Polyurethane adhesives were prepared from a variety of polyether and polyester diols and pure MDI in an NCO:OH ratio of 2.5. These materials were tested as adhesives for two polyethylene substrates and for steel after full cure. It should be realized that these tests were performed to quantify the difference in adhesion of the PU resins to polyethylene for the various polyester and polyether diols, and neither adhesion promoters nor blends of polyester and polyether diols were used to optimize adhesion.Figure 6 shows the adhesion of dimer-based polyurethanes on untreated polyethylene in comparison with systems based on adipate ester and PTMEG, and Figure 7 shows the adhesion on steel. The graphs show that introduction of dimerized fatty acids into PU adhesives results in a substantial improvement of the adhesion to polyethylene. Surprisingly, the adhesion on polar substrates such as steel is similar to or better than the adhesion of polyurethanes based on other polyols. This could be due to phase separation processes that take place in the adhesive during cure; the segments with most affinity for the substrate will move toward it, thus increasing the bond strength regardless of the polarity of the substrate.
Polyurethane Dispersions
Though the coating industry has benefited from solvent-containing polyurethanes coatings, a change to polyurethanes for low-solvent to solvent-free dispersions is necessary. These polyurethanes are used as single binders or in combination with acrylates or styrene acrylates in various coatings and adhesives applications, including anti-corrosion coatings; coatings for wood, plastic and textiles; printing ink; and adhesives.Polyurethane dispersions have acquired an importance in these areas of application in spite of their comparatively high raw material price. Following are benefits of polyurethane dispersions.
- Good environmental compatibility, being aqueous (in some instances they contain small quantities of solvent such as N-methylpyrrolidone)
- Can be processed as one-component systems (in some cases chemical cross linking may be possible or necessary)
- High mechanical resistance, abrasion resistance, flexibility, yellowing resistance (dependent on raw materials), adhesion and impact resistance
Absence of external emulsifiers
There are also properties that can be improved, such as hydrolytic stability, ethanol resistance and UV resistance. Because a large percentage of polyurethanes consist of polyols, isocyanates and chain extenders, it is to be expected that polyols have a major impact on the above-mentioned list.Dimer Acid for Polyurethane Dispersions
Dimerized fatty acids are expected to have hydrolytic stability, mechanical strength and adhesion. Because dimer fatty acids are hydrophobic, it is challenging to get them into water.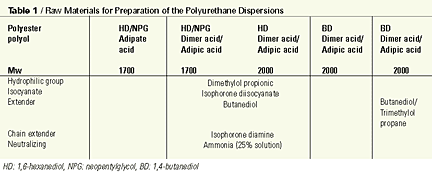
Preparation of Polyurethane Dispersion
The polyurethane dispersions were prepared making use of the so-called acetone process: the addition reaction between the dimer-based polyester polyols, dimethylolpropionic acid and isocyanates that take place in the presence of water-dilutable solvents such as acetone or methyl isobutyl ketone. The formation of the prepolymer takes place in the organic phase, and after the theoretical NCO content has been reached the carboxyl groups are neutralized with a tertiary amine. After further chain extension, the polymer is transferred into water. Once successfully dispersed into water, the low-boiling solvent is removed by gentle distillation, to give a solvent-free polyurethane dispersion with a solid content of approximately 40%.For our preparation of the polyurethane dispersions, an experimental dimerized fatty acid-based polyester polyol was used. Table 1 shows the raw materials used to prepare the polyurethane dispersions.
The resulting polyurethane dispersions, having a solids content of 40% and a particle size in the range of 100-200 nm, were freeze-thaw stable for more than one month using a cycle of 11 hours at Ð5 deg C followed by 11 hours at 40 deg C.
Mechanical Properties
The mechanical properties that were evaluated were: elongation, hardness and adhesion.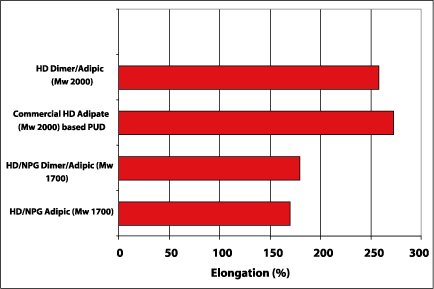
Elongation
The elongation of the produced polyurethane dispersions was evaluated. The test samples were put on 500 m wet on glass and were thickened by a rheology modifier (TAFIGEL PUR 40 from Manzing Chemie). Figure 8 shows that the elongation of dimer fatty acid-based polyester polyols with Mw 2000 is in the range of the commercial-based HD adipate-based polyurethane dispersion. The polyester polyols with Mw 1700 yield lower elongation due to less strain hardening.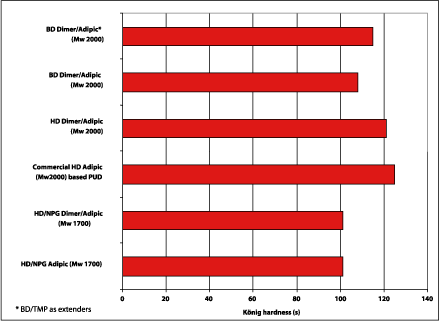
Coating Hardness of the Polyurethane Dispersions
The structure of the dimer fatty acid offers flexibility, which normally means a reduction of coating hardness if the coating is not reformulated. For polyurethane coatings based on the dimer fatty acid-based polyols, hardness was measured with Konig and pencil hardness. The Konig results are shown in Figure 9, where the dimer fatty acid-based polyester polyols (Mw 2000) are comparable to the commercial PUD.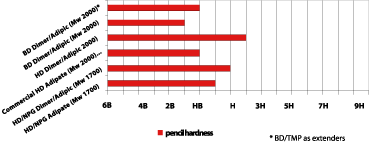

Adhesion to Various Substrates
Evaluating the cross-cut adhesion of the polyurethane dispersions on a range of different substrates shows that the polyols based on HD/NPG with a Mw of 1700 fail in the adhesion to acrylate foil. The dimer fatty acid-based polyesterpolyols with Mw 2000 have-improved on PVC and ALU-foil over the commercial-based polyurethane dispersion (see Table 2).Dimer fatty acid-based polyester polyols offer good mechanical properties and good adhesion to PVC, aluminum and steel.
Chemical Resistance and Water Uptake
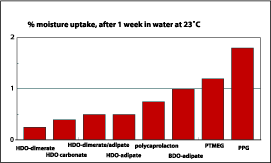
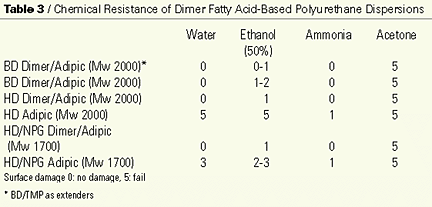
Table 3 shows that the chemical resistance of the dimer fatty acid-based polyester polyols in the coating application is overall good. The most striking is the water resistance when the HD/NPG adipate is compared with the HD/NPG dimer/adipic-based polyester polyol. The dimer fatty acid-based polyol shows no damage to the surface, while the adipate has severe damage to the surface. With these results in mind, the water uptake of polyurethane films was tested. Figure 11 shows that the hydrophobic character of the dimer fatty acid has a positive impact on the water uptake. The adipate based polyurethane films take up to 9% of water vs. only 2-3% for dimer fatty acid-based polyols. The dimer fatty acid-based polyols stay below 10% water uptake when the same test preformed at a higher temperature.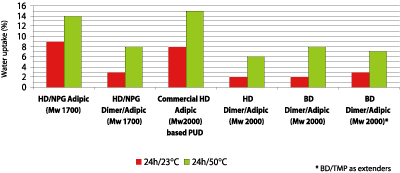
Heat, Oxygen and Ultraviolet Radiation Attack
In comparison with the used polyether-type polyols, dimer fatty acid-based polyesters offer lower sensitivity to heat, oxygen and UV radiation, all of which attack ether bonds. The performance of dimer fatty acid-based polyurethanes was compared with an adipate polyester type and PTMEG diol in a Weather-O-meter experiment (see Figure 12). The polyether formulation breaks down quickly because of UV attack. The adipate polyurethane also fails quickly, which we attribute to the affect of periodic “rain fall” in the Weather-O-meter, causing hydrolysis of the ester bonds. The dimer fatty acid-based polyurethane resists hydrolytic attack as well as degradation by radiation and oxygen.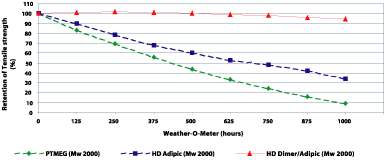
Conclusion
It has been demonstrated that polyester polyols based on dimerized fatty acids offer unique properties. Especially useful is the combination of hydrolytic stability and resistance against attack by heat, oxygen and UV radiation. The dimer technology produces materials with good mechanical properties and good adhesion on PVC, aluminum and steel. Furthermore, an overall good chemical resistance with a fair to good resistance against ethanol compared against a commercial HD adipate-based polyurethane dispersion is produced.With respect to water uptake and resistance to hydrolysis, the dimer fatty acid-based polyols exceeded the adipate-based polyols. Adding to that the inability to strain-harden makes the dimer-based polyester polyols extremely suitable for flexible substrates.
This article is based on a paper presented at the 30th International Waterborne, High-Solids, and Powder Coatings Symposium, February 26-28, 2003, in New Orleans. Symposium sponsored by The University of Southern Mississippi School of Polymers and High Performance Materials.
For more information, contact Erwin Honcoop and Eric Appelman, PO Box 2, 2800 AA The Netherlands.




Report Abusive Comment