
Exempt solvents are not new; from the very first, both federal and state regulators have excluded certain solvents from consideration as VOCs. Initially, such exemptions were of little practical value for paint formulators — early exemptions were mainly granted to halogenated products such as methylene chloride and methyl chloroform, low boiling materials not generally used in coatings compositions. The very fast evaporation rate of these materials, perhaps as much as anything else, restricted their use in coatings. Excessive use of low boilers with fast evaporation rates produces severe flow and leveling problems in both atomizing and spreading application techniques. Methyl chloroform was also an ozone depleter and not a good solvent for coatings.
Fortunately, over the last seven years or so, a few more practical coating solvents have been exempted from categorization as VOCs by the EPA.1 Unlike the chlorinated materials noted above, none of these materials are either ozone depletors or HAPs. As VOC-exempt solvents, these materials may be used in coatings, but are excluded from the weight aggregation of total VOC although, like water, their volume must also be excluded from the VOC calculation. In effect, these solvents may be added to a given formulation without restriction, reducing viscosity and, therefore, improving application without affecting the VOC content of the formulation. They may even be added with impunity by applicators in the field.2 The exemption, of course, does nothing for volume solids, which are progressively reduced with exempt solvent additions.
Deregulation Solvents - Parachlorobenzotrifluoride (PCBTF)
Some of the first materials de-listed as VOCs by the EPA were chlorofluorocarbons, many of which were, like methylene chloride, too low boiling to engender much interest by coatings formulators. Parachlorobenzotrifluoride (PCBTF), however, was low enough in volatility and a good enough solvent for coating resins to be of practical value to coatings formulators. PCBTF was delisted in October 1994. Unlike methylene chloride and methyl chloroform, this relatively dense solvent was new to the coatings industry,3although over the past seven years or so its usage volume in coatings has increased many hundred fold in spite of its high price tag. (Because of the greater volume of this material now being regularly consumed by the coatings industry, among others, Occidental Chemical, the manufacturer of PCBTF has recently implemented a significant price reduction). PCBTF has been adopted by the coatings industry because of its unique position among the growing list of deregulated materials as a reliable, medium boiler of good solvency for a very wide spectrum of coating resins. If parachlorobenzotrifluoride is not as broadly efficient a solvent as other delisted materials (acetone), it is sufficiently so in most of the newer, low-molecular-weight polymers (alkyds, epoxies, urethanes, polyesters, even acrylics) so that most formulators find significant utility in compositions based on these systems. Unlike solvents such as acetone, PCBTF is essentially inert. Atypical of other chlorinated solvents, it is stable against dehydrochlorination, so that it may be safely used in coatings for aluminum, even when those coatings are baked. Unlike several other exempts, it will not react with hydroxyl groups, amines, epoxies, isocyanates or acids, so that it has greater utility in coating applications. It may, for example, invariably be safely used with either polyol or isocyanate in polyurethane formulations, and in epoxies with either the epoxy resin or the amine without affecting cure, dry time, stability or viscosity. In thermoplastics, PCBTF is somewhat less universal than acetone but, when used in combination with acetone and/or strong ester, ketone or aromatic tails, it has been successfully employed in nitrocellulose, vinyl chloride/vinyl acetate and chlorinated rubber lacquers. In many acrylics and high styrene systems, PCBTF will serve alone, and it is slow enough to give good defect free films without the need of other solvents. Because of its cost, however, PCBTF is most usually used as a medium boiling solvent or diluent combined with acetone and/or other low boilers together with strong high boiling tails. Because PCBTF is a halogenated compound, it is much less flammable than might be expected from its evaporation rate. Boiling in about the same temperature range as xylene (280ºF), for which it is an excellent alternative, its flash point is some 30ºF higher than the aromatic. Thus, PCBTF is classified as a combustible material by OSHA and is not regulated by DOT because it does not sustain combustion (49CFR173 170).Acetone
Shortly after PCBTF was delisted, the EPA also delisted acetone as a VOC. While this solvent has much the same evaporation rate as methyl chloroform, it is a far stronger solvent for almost all coating resins and, as such, is considered a primary low boiling reducer for a variety of coating types. This, along with its low cost, are the primary advantages for acetone. Aside from its flammability (TCC flash point -4ºF) and an extremely fast evaporation rate which severely limits the quantity of acetone which may be practically employed in any given formulation, its main drawback is its ketonic reactivity (hydrogen acceptance) with certain mainstream binders (epoxy curing agents). Its very low boiling properties also result in a tendency to induce blushing and may cause problems with urethane systems. Ketonic residues retained in an applied film may also detract from that film’s weatherability.
Methyl Acetate
Methyl acetate has also been recently exempted. This ester is also less well known among coating formulators, although it has been available for many years. In many respects, methyl acetate resembles acetone as a solvent for coatings. It is not quite as fast and somewhat more dense, but it is marginally less flammable (TCC 15ºF) and not quite as strong.Like all of the esters, methyl acetate is, however, subject to hydrolysis especially at extremes of pH, and will, in the presence of amines, undergo slow aminolysis, so occasioning loss of functionality in epoxy curing agents. It is also more expensive than acetone, and not sufficiently different as a solvent to make it really attractive to most formulators.
Tertiary Butyl Acetate (TBAc)4
Fortunately, a second ester, tertiary butyl acetate (TBAc), is now under consideration for VOC exemption. It is expected that this new solvent will soon be added to the tools available to the modern formulator. While TBAc inevitably suffers from the same reactivity disadvantages that affect methyl acetate (hydrolytic sensitivity, the potential for aminolysis in epoxy curing agents, etc.), its exemption will be most welcome. This is another valuable addition to the growing list of permissible solvents that relieves many of the restrictions that formulators have now come to accept as a fact of life. Tertiary butyl acetate is a solvent of about the same overall strength as PCBTF (in some systems such as chlorinated rubber, it is a better solvent than PCBTF; in others, such as epoxies, it is a poorer solvent). It is, however, somewhat faster than PCBTF, having much the same evaporation rate as toluene or ethyl alcohol. It is, in consequence, a red label solvent having a flash point of 60ºF (considerably lower than the flash point of PCBTF). While TBAc has probably somewhat better hydrolytic stability than methyl acetate, at extremes of pH it will hydrolyze to tertiary butyl alcohol and acetic acid (both VOCs). Hydrolysis may also limit its applications in metallic pigmented systems including zinc rich primers and aluminum containing coatings. Careful testing may be wise in strongly acid systems or systems bearing basic pigments.
The Volatile Methylsiloxanes
Unfortunately, there are no exempt high boiling solvents that have true value for the coating formulator. Several high boiling, volatile methyl siloxanes5 are available, but these materials generally have minimal solvency for mainline binder systems. (They may be useful with certain silicone-based coatings and even with some long oil alkyds.) The author has experienced problems in using these solvents in epoxy ester based coatings, however. The volatile methyl siloxanes cannot, therefore, be considered as primary high boiling tails for most high-performance coating systems. As such, high boilers must be especially good solvents for the binders in which they are used. The volatile methyl siloxanes are also high in price.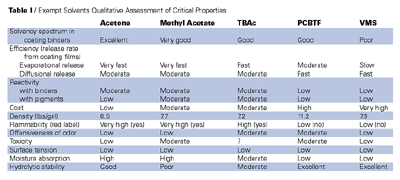
Formulating Strategies
Because of the lack of VOC-exempt strong high boilers (see Table 1), formulating strategies employing these various exempt solvents must generally rely on non-exempt high boiling tails. Such solvents include the high boiling aromatics, methyl amyl ketone, cyclohexanone, isobutyl isobutyrate, several high boiling alcohols, glycol ethers and glycol ether acetates to complement a solvent system made up primarily of low and medium boiling exempts. In any given exempt formulation, these high boiling solvents make up most, if not all, of the non-exempt solvents. In addition to these high boiling tails, acetone, tertiary butyl acetate, and parachlorobenzotrifluoride can be employed in various ratios depending on the binder and the coating type to complete the solvent system. Although this type of strategy is a boon to formulators and applicators who wish to apply aesthetically pleasing coating films with traditional performance profiles, there are inevitably pitfalls, which are discussed below.

Pitfalls - In Thermoplastics
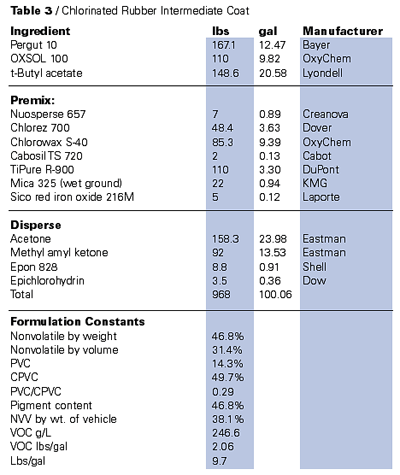
Until the deregulation of the aforementioned solvents, the high-molecular-weight thermoplastics that relied on the use of large quantities of solvent were rapidly becoming a virtually dead item. Both coating manufacturers and the manufacturers of the resins were aggressively abandoning these technologies. The thermoplastics were indispensable and widely used under special exemptions in relatively specialized applications, because of either aesthetic or performance requisites. An example is high end furniture finishing, where nitrocellulose lacquers are still a primary coating type.On the other hand, there are no domestic suppliers of chlorinated rubber left, although these resins are still available from foreign sources. Once widely employed as valuable maintenance binders, the vinyl chloroacetates have also been hard hit by VOC limitations.
It is, however, possible using the exempt solvent systems based on the above noted strategies to deliver applicable thermoplastics based on most high-molecular-weight polymers at significant VOC reduction.6 Tables 2 and 3 demonstrate these techniques with both vinyl chloroacetates and chlorinated rubber. The deregulated acetates and acetone are also good solvents for ester-based polymers such as nitrocellulose and, along with PCBTF as a diluent, will significantly reduce the VOCs of this type of system.
In Alkyds and Polyesters
Changes to solvent systems that result in significantly lower VOCs are also possible with the saturated polyesters and with oxidizing systems such as alkyds, epoxy esters, and oil modified urethanes. The introduction of both tertiary butyl acetate and PCBTF into these systems, replacing solvents such as xylene and mineral spirits, is not a difficult task. Actually, these lower molecular weight dry film precursors should be amenable to even lower VOC systems than have as yet been achieved. Far lower VOCs would be possible if the manufacturers of the alkyds and other resins would offer VOC-exempt versions of their resin solutions. Most of the traditional resin systems of this type were carried in aliphatic (for longer oil length oxidizing systems) or aromatic hydrocarbons (for polyesters and the shorter oil length alkyds). More recently high-solids versions of these resins have been available in oxygenated solvents such as n-butyl acetate, the medium and high boiling ketones, and the glycol ethers. Although TBAc is somewhat faster than all of these materials, it should be possible to produce solutions in this solvent. PCBTF alone or in combination with TBAc with or without non-exempt solvents could replace many of the higher boiling solvents in both high-solids and conventional resins.In Epoxies
The production of low-VOC two-component epoxies has been one of the least taxing accomplishments of the formulator of modern coatings. This has been mainly attributable to the substitution of low-molecular-weight liquid bisphenol A epoxies for the more traditional medium-molecular-weight resins. New lower molecular weight amines and polyamides having higher equivalent weight facilitate these efforts, especially where benzyl alcohol is employed as a non-reactive diluent. This diluent appears to have great affinity for the epoxy system, and does not readily volatilize from the film in the EPA Method 24 (ASTM D-2369) test, acting at least in part as a non-reactive, non-volatile diluent. Again, both monofunctional and difunctional epoxy diluents may also be employed in epoxy systems to further reduce viscosity for low VOC.Unfortunately, many of the solvents used in even these low VOC systems are regulated as HAPs materials (xylene, MEK, MIBK, glycol ethers, etc.). These solvents are of increasing concern to formulators and users of coatings, as well as to regulators. As such, while low-VOC systems are possible, even without using exempt materials, there are, therefore, still pressures on the formulator for further reformulation. In developing epoxy formulations, for example, PCBTF finds valuable utility as part of the solvent system in both the epoxy and the amine component. It fulfills this role with no untoward effects that relate to viscosity.
Care is necessary with the other exempt materials, however. Acetone, like other ketones, forms ketimines with most amines and profoundly influences cure. This may be advantageous or severely disadvantageous. Whatever, the impact of acetone usage must be understood and anticipated, and the solvent, therefore, employed judiciously. The extent of cure retardation depends upon into which component the acetone is introduced and when. Introduction of this solvent into the amine component by the manufacturer as part of the curing agent component allows the ketimine to become well established long before the system is eventually mixed in the field and applied, and so has the most retarding effect on cure. In this case, the ketimine must first dissociate before the amine becomes available for the amine/epoxy reaction to proceed. (As dissociation requires the presence of water, most usually atmospheric water, cure rates may be effected by relative humidity. Unblocking will occur progressively more easily at relative humidities above 30%).
Alternatively, if the acetone is used in the epoxy component no reaction with the amine will take place until the components are mixed in the field. At that time the acetone/amine reaction will compete with acetone loss by way of evaporation as well as with the epoxy/amine reaction. The effects of the solvent on cure retardation will, therefore, be less, though depending upon temperature and relative humidity, still substantial. (The acetone/amine reaction occurs more rapidly than does the epoxy/amine reaction with most curing agents.) Under certain conditions (low temperature and high humidity that retard the epoxy/amine reaction but favor the acetone/amine reaction), the use of acetone (without appropriate allowance for adequate induction) may lead to amine blush and carbamate salt formations in some epoxy systems. This can lead to ugly discoloration, loss in gloss and even recoating problems.
Care must also be used in the placement of the VOC-exempt ester solvents in epoxy formulations.7 Their incorporation into the amine component should be avoided. Over time, ester-based solvents of this type may slowly react with the amine curing agent, undergoing aminolysis and converting the amine to an amide, while simultaneously being themselves converted to the alcohol. This results in loss of functionality of the amine, and progressive loss of potential cure. The reaction requires time and is probably of little consequence where the ester is packaged with the epoxy resin, stored at normal temperatures, and exposed to the amine only after mixing in the field. Where the ester is packaged with the amine and sits on a shelf over a period of months, however, the effect on crosslink density may be more profound. In cases where VOC exempt esters are used in the epoxy component, and the component is stored at elevated temperatures over any prolonged length of time, reaction is also possible between the hydroxyl groups of the epoxy and the ester. This results in the formation of an epoxy ester and the conversion of the acetate to the resultant alcohol (i.e., either methanol or tertiary butanol) in the case of the exempt esters. This reaction is more likely with the more highly hydroxylated higher molecular weight epoxies than it is with the liquid epoxy resins, which are favored for low VOC systems.
In epoxy systems, solvency can be more of a problem than with other systems, especially as the molecular weight of the epoxy resin increases. Neither PCBTF nor tertiary butyl acetate is a good solvent for the medium- to high-molecular-weight epoxy resins. However, in combination with acetone and butanol, PCBTF has been successfully employed in conventional epoxy/polyamide metal primers such as MIL-P-233778, with the PCBTF replacing the ketones, aromatics and lower molecular weight alcohols. Interestingly, when a portion of the PCBTF in this primer was replaced with tertiary butyl acetate, precipitation of the resin system was encountered. Problems occurred only after the epoxy was catalyzed with the PCBTF solvated amide just before application. The unmixed components seemed quite stable.

In Urethanes
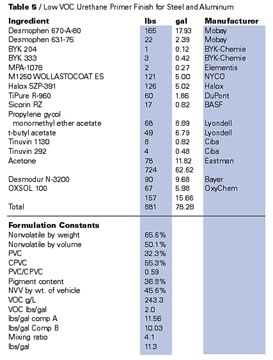
Only slightly more difficult to formulate for truly low VOCs are the two-pack polyurethanes. Many polyisocyanates, isocyanurates, uretidiones, etc., are low enough in viscosity to be used as the curing agent of these two-pack systems without solvents. In the isocyanate, the role of any solvent is usually as a device to balance volumetric apportionment to user-friendly ratios (4:1 etc.). In this application, parachlorobenzotrifluoride is admirably suited, for the solvent neither reacts with the isocyanate (for which it is an excellent solvent) nor does it tend to attract water into the system as do hygroscopic acetates and ketones. Isocyanate solutions in PCBTF (prepared with and without a nitrogen purge) have shown no instability after nine months shelf storage in the laboratory. Tertiary butyl acetate is somewhat less desirable in this role not only because of its hygroscopicity but because of the potential for reaction of the isocyanate with the products of its hydrolysis.Most acrylic and polyester based polyols are at this time available in either high boiling ketones or esters such as PMA or butyl acetate. Both PCBTF and TBAc have been used as solvents for many of these polymers and these strategies should do much to further reduce VOCs. Similar solvents (ketones, esters and aromatics) are used by coatings manufacturers to reduce these resins, and all of these materials are replaceable (or partially replaceable) with the exempt offsets. Certain polyols, both acrylic and polyester, are available as 100% or virtually 100% solids systems and, using these materials with exempt solvent, it may be possible to approach zero-VOC solvent-based 2K polyurethanes.
Table 4 shows an example of such a product. The solvent blend in this formulation is important. Although tertiary butyl acetate appeared to be a slightly better solvent for the acrylic polyol than did parachlorobenzotrifluoride, problems were encountered when levels of tertiary butyl acetate exceeded 5–10% of the total solvent system and high film thickness (three mil films and more) was applied. It was found that application of films of much more than two dry mils of the product shown in the table tended to result in severe CO2 bubbling, especially when applications were made in humid weather. This effect is believed to be caused by a degree of hygroscopicity in the tertiary butyl acetate. If the solvent is left open to the air (even in an air-conditioned laboratory overnight), water pick up is sufficient to initiate color change in the desiccant activated silica gel (MIL-D-3718A). Thinner film coatings of the two pack urethane noted in Table 4 appear to pose less of a problem even at the higher levels of tertiary butyl acetate.
The Future
Regulatory trends, in so far as VOCs are concerned, remain at this time in a state of considerable flux. Changing regulations almost always mean reformulation, and the continuing rethinking and refinement of controls by the regulators are inevitably difficult and costly for the paint manufacturer. The earliest controls (Rule 66) primarily addressed those solvents known to have highest reactivity. Later regulations, first considered all solvents as being equally culpable, but later adopted a reactivity threshold control that employed ethane reactivity as a bright line. Solvents, having less reactivity than ethane, were exempted as VOCs; use of those solvents having more reactivity was restricted. This had the effect of forcing formulators to employ more of the less reactive materials in lieu of the more reactive species. In fact, of course, all solvents have some atmospheric reactivity and the viability of the ethane reactivity “bright line” used to differentiate exempt and non-exempt solvents is, in some circles, now being challenged. It seems likely that in the long run, a system of controls aimed more specifically at regulating each solvent on its own merits and in direct response to its own reactivity will be established. This will allow higher levels of the less reactive solvents to be used, and lower levels of the more reactive species. Although these trends will greatly complicate both formulation and enforcement, it seems doubtful that evolving rules of this type will greatly affect the type of formulation strategy that has resulted from the present exempt/non-exempt controls.
Although it is conceivable that future controls may be reimposed on materials such as acetone, parachlorobenzotrifluoride and the acetates, allowable usage of these materials will inevitably continue to be much greater than the permissible usage of the more reactive species. This is especially the case with HAPs such as MEK, MIBK, toluene and xylene. Those formulations that favor the use of acetone, PCBTF, tertiary butyl acetate, etc., none of which are either ozone depletors (ODS) or HAPS, can be expected to remain viable even in the face of these potential refinements in the present regulations. Thus, it seems reasonable to expect that reformulations made today, under the present controls, will most likely serve well for the most reasonable control strategies to be used as the new century unfolds.


Report Abusive Comment