All of these technologies allow coatings to be cured in place using specialty acrylics esters — acrylate and methacrylate monomers and oligomers.
Advantages Of Cure-In-Place Technology
Three main advantages of cure-in-place technology with specialty acrylics are: low to zero VOC content, improved performance, and significantly decreased drying times for paper, wood, metal, and plastic substrates.Rapid Cure Speeds
Cure-in-place technology reduces cure times to nanoseconds (as in the case of UV and EB curing), to a few minutes or hours, using peroxide and azo thermal systems or Michael Addition reactions.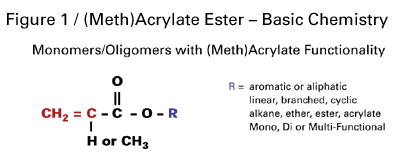
High Performance
Cure-in-place technology generally uses acrylate- and methacrylate-terminated monomers and oligomers. The monomers and oligomers provide a variety of building blocks to “tailor-make” coating systems. The general structure for these components is shown in Figure 1. Polymerization takes place through addition across the C=C unsaturation in the acrylate or methacrylate group.The variety of available specialty acrylic esters enables formulators to improve the right performance properties for almost any application. Coatings varying from hard- and scratch-resistant to being flexible and exhibiting high elongation can be developed with today’s selection of specialty acrylic esters.
Low VOCs
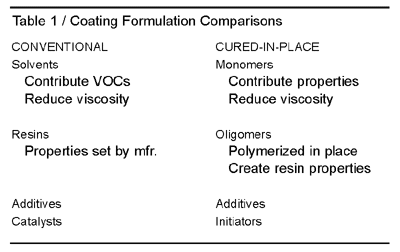
Cure-in-place technology can include a variety of low-VOC materials, in addition to acrylate and methacrylate esters. Allyl derivatives, N-vinyl derivatives, low-molecular-weight epoxies, and acetoacetate derivatives are some examples. The basic formulating differences between conventional coatings and coatings developed for cure-in-place technology are shown in Table 1.Formulators of conventional coatings have been under continuous pressure from the EPA to decrease VOCs in their formulations. Cure-in-place coatings containing acrylic esters not only offer decreased cure times, but also can be formulated to 100%-solid systems, eliminating the need for solvents altogether.


Monomers
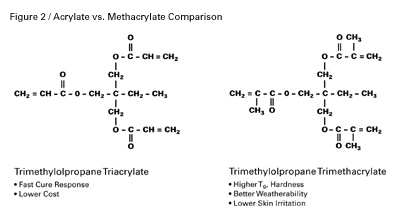
Monomers are generally low-viscosity acrylates and methacrylates that function as reactive diluents, crosslinkers, and performance-property enhancers. Their viscosities can range from 5–25,000 centipoise. They are lower in molecular weight than oligomers and vary in functionality. Mono-, di- and tri-functional monomers, shown schematically in Figure 3, are the most common, but functionalities as high as five are available to formulators.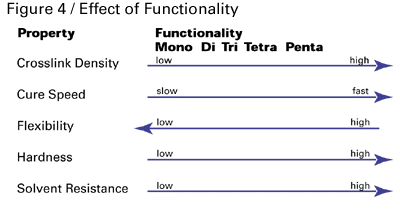
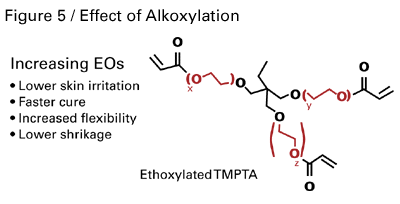
Low Skin Irritation
First-generation monomers developed for UV and EB curing often were skin-irritants and not well suited for spray applications. However, specialty acrylates have skin irritation levels of less than one on the Draize scale. Typically, this is achieved through the alkoxylation of monomers. In addition to lower skin irritation, the ethoxylation or propoxylation of acrylates also imparts other advantages, such as faster cure speed, better flexibility and higher impact resistance. The structure and properties provided by ethoxy groups are shown in Figure 5. The alkoxylation of monomers has brought a substantial improvement in safety and handling properties and has broadened the markets for radiation-curable coatings. Monomer chemistry affects many other coating properties, such as resistance to abrasion, adhesion and heat. One measure of a coating’s ability to withstand heat is its glass-transition temperature (Tg).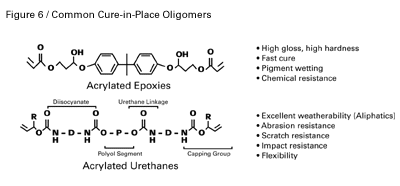
Oligomers
Oligomers are higher-molecular-weight (1,000–30,000) cure-in-place coating components used as the base material that determine the major physical properties. Oligomers are based on a variety of chemistries including acrylated urethanes, epoxies, polyesters, and acrylics. Figure 6 depicts the general structure of the two major types used in cure-in-place coatings — epoxy acrylates and urethane acrylates.
Free-Radical Cure-In-Place Technology
In free-radical cure-in-place technology, as shown in Figure 7, some type of initiator is required for monomer combination or addition to take place and for polymerization to occur. The type of initiator depends on the cure method.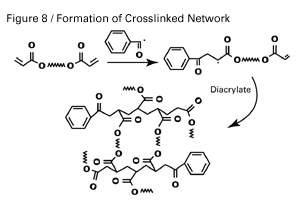

Radiation Curing
In UV-cured coating systems, a free radical-generating photoinitiator is required. Radicals are typically produced by hydrogen abstraction upon exposure to UV light. Common photoinitiators include benzophenone, benzil dimethyl ketal and 2-hydroxy-2methyl-1-phenyl-1-propanone.In EB curing, sufficient energy is produced from electron bombardment of the coating to generate free radicals without an initiator. Radiation curing offers the following benefits.
- 100% reactive single component systems.
- Fast cure (dry times < 1 second).
- Increased line speeds (up to 1,000 fpm).
- Ability to coat heat-sensitive substrates.
UV/EB curing is currently used in a variety of high-performance coating applications such as topcoats for vinyl and wood flooring, metal and wood furniture, overprint varnish (OPV) for plastic and paper packaging and coatings, circuit board coatings, and coatings for plastic and metal containers.
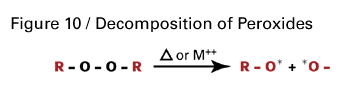
Thermal Cure
Free radical cure-in-place coatings using specialty acrylic esters can also be formulated using temperature-sensitive initiators such as peroxides or azonitrile compounds. In these systems, radicals are generated from decomposition of the initiator by heat or by reducing agents like metal driers at ambient temperature, as shown in Figure 10.The selection of the peroxide is dependent on the temperature at which the coating will be cured and the required shelf life of the coating. The key property is its half-life temperature or the temperature at which half of the radicals are generated in a certain period of time.
Commonly used peroxides are methylethylketone peroxide, cumene hydroperoxide, t-butyl perbenzoate, and 1,1-di-(t-butylperoxy)-3,3,5-trimethylcyclohexane. The selection of peroxide determines whether the coating will be a single-component or a plural-component system.
An important consideration when formulating peroxide-cure coatings is that two competing cure mechanisms occur. At the coating surface, where oxygen is present, aerobic cure takes place; below the surface, where no oxygen is present, anaerobic cure occurs. The anaerobic cure below the surface is much faster than the aerobic cure since oxygen is a good free-radical scavenger. Therefore, the key to achieving uniform and complete cure is to eliminate oxygen at the surface either by mechanical means such as inerting the atmosphere with nitrogen, or by chemical means through the use of metal driers and monomers that are a good oxygen scavenger, such as allylic functional monomers.
Peroxide cure-in-place coatings offer the following benefits.
- 100% reactive systems with higher film builds.
- Isocyanate-free, formaldehyde-free systems (low toxicity).
- Low-temperature curing (down to 0°C).
- Use of conventional application and drying equipment.
Peroxide cure-in-place coatings using acrylic esters are currently used in high-performance automotive primers and topcoats, coatings for concrete bridge decks and industrial flooring, and specialty coatings for wood and paper.
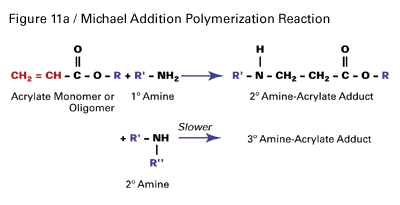
Michael Addition Cure-In-Place Technology
Acrylic esters can also be used as reactive diluents and modifiers in other cure-in-place coating technologies, such as two-component epoxy systems cured with polyamines. In these systems, the amine-curing agent is more of a co-reactant than an initiator or catalyst. Acrylate esters can react with an amine through a Michael Addition reaction, as illustrated in Figure 11a.
Acrylate esters can be reacted with a number of amine curing agents such as aliphatic amines, cycloaliphatic amines, amidoamines and polyamides. However, aliphatic amines are preferred due to their abundance of primary amine hydrogens. The most effective amine curing agents are based on diethylenetriamine, triethylenetetraamine and tetraethylenepentaamine. Addition of an acrylate ester to an epoxy cure-in-place coating offers the following benefits.
- 100% reactive systems.
- Viscosity reduction without loss of properties.
- Fast ambient, sub-ambient cure without use of mercaptans.
- Reduced amine blush.
Michael Addition cure-in-place technology using acrylate esters is currently used in epoxy traffic paint, epoxy coatings for concrete such as secondary containment and industrial flooring, and epoxy-based protective coatings such as tank linings and corrosion-resistant primers.

Conclusion
In conclusion, cure-in-place technology allows the coating formulator to:- Develop 100% reactive systems, which are solvent-free.
- Achieve faster dry times at lower temperatures.
- Reduce viscosity without a loss of performance.
The use of acrylate and methacrylate ester monomers and oligomers provides the following.
- Building blocks to “tailor-make” coating resins
- A choice of curing technologies to fit the application, such as UV/EB, peroxide/azo systems, or polyamines in the case of Michael Addition reactions
- Tools to meet the demands of compliance and higher performance.


Report Abusive Comment