Amines and amine derivatives are the most diverse group of epoxy curing agents. The fully polymerized epoxy resins exhibit a very wide range of thermal and mechanical properties. Though other classes of compounds (e.g., anhydrides, phenolic resins and Lewis acids) are used as hardeners for some applications, the breadth of performance imparted by amine hardeners is unmatched. This paper provides an overview of the wide variety of amine hardeners, including several recent developments that can expand the capabilities of epoxy formulators into new applications.
History
Amine compounds were among the earliest reactants used with epoxy resins to produce useful products. As epoxy resins became more widely available following their development and commercialization in the late 1940s and mid-1950s, utilization of an ever-wider variety of amine compounds ensued. Because most amines are reactive at room temperature with epoxy resins, such formulations are typically provided as two separate parts (or "sides"), which are mixed just prior to application.
Though a variety of epoxy resin products are commercially available, liquid resins based on the diglycidyl ether of bisphenol A (also termed DGEBA or BADGE type resins) have the widest use and availability due to their relatively low price, which is partially gained from economies of scale. Because of this, the epoxy portion of epoxy formulations often remains relatively fixed, and most variations in processing and performance are obtained by making changes to the hardener side of the formulation. The wide variety of commercially available amine compounds and decades of study and formulation have helped to make this group of hardeners the most versatile and widely used of any epoxy reactants.
Amine/Formulation Choices
As mentioned previously, the choice of epoxy resin can be used advantageously to affect some processing, thermal and mechanical properties, but the wide diversity of amine curing agents typically allows the greatest latitude in creating formulations to fit a wide variety of application needs. In this article, unless otherwise stated, "epoxy" will refer to a standard DGEBA type resin having an epoxide equivalent weight (EEW, or WPE) ranging from about 182-192 grams per epoxide equivalent.
The three main use criteria for creating or choosing an amine hardener (or blend) for an epoxy formulation are (in no particular order): cost, processing requirements and performance requirements. These will be addressed in sequence.
Amine Cost vs. Value
When choosing between hardeners that have nominally similar processing and performance characteristics, one should not simply go by price alone, but rather, do a calculation, taking into account the cost per pound (or perhaps, unit volume) of the final, cured material. As an example, such a calculation would reveal that a more "expensive" amine hardener "A" ($2.40 per pound), having an amine hydrogen equivalent weight (AHEW) of 44, results in a cheaper system cost than a corresponding "cheaper" amine "B" ($2.25 per pound) having an AHEW of 60, since more of the amine B must be used due to its higher AHEW. A similar argument would apply to other, non-amine hardeners.
Processing Requirements
Stoichiometry and Mix Ratios
As a general rule, use of a 1:1 stoichiometric ratio of amine hydrogen to epoxide groups will, when fully reacted, ensure maximum stability of the product. Such a stoichiometry may not, however, always provide the most desirable processing characteristics or combination of particular properties. For some formulations, being either off-stoichiometry (i.e., not a 1:1 equivalents ratio) or not achieving full reaction, provides an increase in some properties. In these instances, other properties, less important for a given application, may be sacrificed. Being off-stoichiometry and/or under-cured, can lead to higher modulus, higher density, greater hardness, more brittleness and a lower glass transition temperature. Resistance to solvents or moisture may also be decreased.
Sometimes the existence of off-stoichiometric formulations is inadvertent, having been caused by the false assumption that formulations based on parts by weight are interchangeable with those based on parts by volume. Switching from parts by weight to parts by volume should not be done without recalculating the component percentages because the densities of most amine hardeners (around 0.92 to 0.98 grams per cc) are considerably lower than the densities of epoxy resins (often 1.15 to 1.20 grams per cc).
Effect of Temperature on Available Processing Time
Because the amine-epoxy reaction is exothermic, larger masses of material (e.g., a gallon can vs. a small jar) will have considerably higher exotherms, and the reaction will proceed faster as the temperature increases. As a result, particularly for the more reactive amine hardeners, application must occur before the end of an ever-shortening pot-life, and the thickness of such applications (such as castings or molded composite parts) must be limited to that which can withstand the resulting temperature rise. Otherwise, the interior of the part may burn itself.
For some applications, the processing time required is very viscosity-dependent, with lower-viscosity systems being more suitable in cases where thorough wetting of fillers, fiber performs, etc., is necessary to ensure good mechanical properties of the cured products. In many such applications, the end of pot-life is signified by some maximum viscosity beyond which the resin will no longer suitably flow into a mold, wet fibers, etc.
Since higher initial mix temperatures promote faster reaction, thereby decreasing gel times, one might also expect the working times to also decrease; however, because flow, permeation and wetting are highly viscosity-dependent, increasing the temperature can decrease the necessary pot-life by more time than the amount of working time that is lost due to the faster reaction. Thus increasing, rather than decreasing, the temperature has been found to be a useful means of increasing the utility of many amine-cured epoxy resin systems.
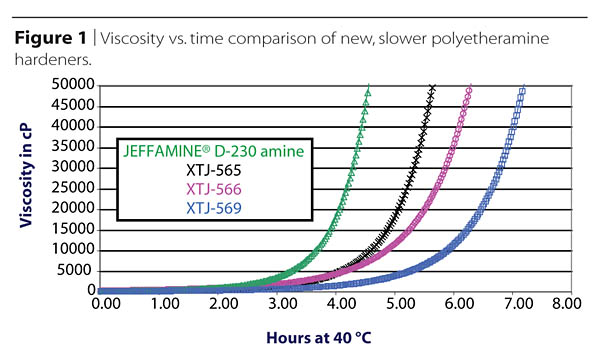
Pot-life vs. Curing Time
Every epoxy user likes plenty of pot-life or processing time, and the available range of amine hardeners fills most of these needs, but sometimes new applications create needs that stretch beyond the boundaries of the available products. As an example, in one relatively recent development, new amine hardeners exhibiting particularly long pot-lives were produced to meet needs created by the molding of extremely large composites. Figure 1 shows an example of the viscosity build vs. time at 40 °C for a few such new materials, compared against a standard, long commercialized polyetheramine known for its particularly long pot-life.
As hardeners providing increased pot-life (and decreased reactivity) are used, curing time, or the time to achieve the desired level of properties prior to putting the part or coating into (or back into) service, is also extended. Though there are some formulation "tricks" that can narrow the time for sufficient curing while still providing a usefully long pot-life, these are outside the scope of the current article. In general, for applications where the applied epoxy cannot reasonably be heated, formulating to obtain faster cure times will come at the expense of shorter pot-lives.
Classes of Amine Hardeners
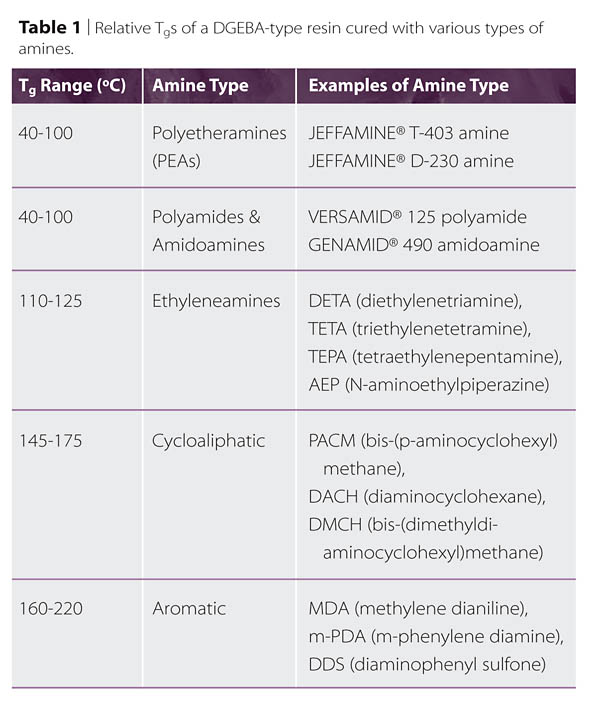
The wide variety of commercially available amines may be grouped by chemical structure. In cases where an amine has a combination of structures, it is sometimes grouped, for convenience, with those amines whose performance or reactivity characteristics it shares. As a starting point, the categories are proposed as indicated in Sidebar 2, and some of their performance and reactivity characteristics are described. It should be noted that the Tg range assigned to each category is intended to indicate the range of fully cured DGEBA-type resins, based on different hardeners within the category. No common cure schedule should be inferred. Hardeners with higher Tg values, such as aromatic amines, may require elevated temperatures to achieve full curing and attain their maximum Tg. Other hardener types, such as Mannich bases, may typically only be cured at ambient conditions, though if they were baked, higher Tgs might be obtainable.
Additionally, some amines such as imidazole and its derivatives are used as catalytic or co-curing agents, as are some guanidine derivatives such as "dicy" (dicyandiamide or cyanoguanidine).
Performance Requirements
For those formulations that have met the necessary cost and processing requirements, the final performance/suitability of amine-cured epoxy formulations will depend upon their mechanical, thermal and physical properties. In this discussion, thermal properties are singled out from other physical properties because of their significant effect on the cured resin mechanical properties. Foremost of concern among the thermal properties of a cured epoxy system is the glass transition temperature, designated as Tg ("T-sub-g").
Thermal Performance
For some applications, such as fiber-reinforced composites, Tg is one of the first measurements made on a cured epoxy, to determine whether the hardener even qualifies for further work. In other applications, such as coatings, many customers never think in terms of Tg, or measure it. Despite the latter, an understanding of the Tg can aid in solving formulation and/or performance problems.
Many properties of plastics vary greatly, depending upon whether the measurement/use temperature is above or below the plastic's Tg. As a result, much of the formulation of epoxy systems involves selecting combinations of different amine hardeners to change the Tg of the cured epoxy, thus modifying its properties. For many blends, use of the Fox Equation can provide a reasonable estimate of the Tg of the fully cured resin. This equation states that the reciprocal of the Tg of the blend is equal to the sums of the weight fraction of each polymer segment times the reciprocal of that segment's Tg. This is illustrated in the following equation for a two-component amine blend:
1/Tg = X1/Tg1 +X2/Tg2
The numerical subscripts indicate the two different amines in the blend and Tg1 and Tg2 indicate the Tg that would be expected for the epoxy resin if fully cured with each individual amine.
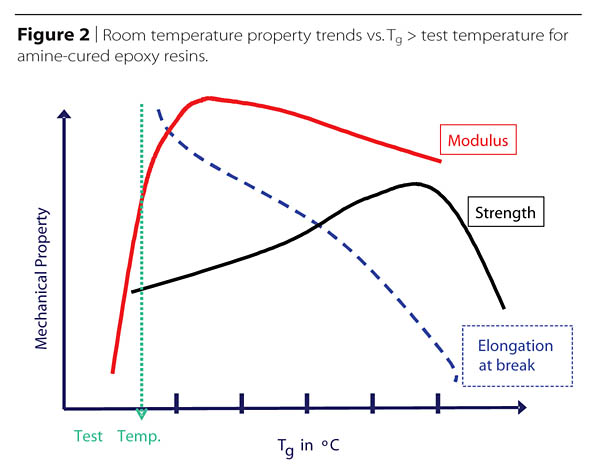
Some Tg value ranges for several different classes of amines are shown in Table 1. Because the Tg is typically taken as the mid-point of a range, when rigidity is important, formulations are typically made so that the Tg attained using the prescribed bake schedule is somewhat higher than the expected service temperature. Furthermore, since environmental exposures may further reduce the Tg, hardeners capable of even higher Tgs may be needed. It is often inadvisable to only target a high Tg since toughness, ‘toughenability', strain to failure, and other properties may decline sharply as the temperature difference between the Tg and the service temperature increases.
Beyond the Tg, other aspects of thermal performance to be considered for some specialized applications are the long-term thermal and thermooxidative stability of the amine-cured epoxies. The performance rankings of different amine hardeners may be quite different when aged at temperatures of 100 degrees C in air vs. nitrogen. Also, degradation pathways proposed to explain high temperature behavior (typically measured in nitrogen), such as those involving formation of free radicals, bear little resemblance to lower temperature thermooxidative degradation.1
Mechanical Performance
The inter-relation of thermal and mechanical performance is a complicated area. Only generalizations of a few of the more important relationships will be mentioned here. Among the important areas of mechanical performance are strength, modulus (related to rigidity or stiffness), hardness and toughness. For most applications, formulations are best chosen to provide glass transition temperatures that are only slightly higher than the expected service temperature. This is done in order to maintain rigidity and surface hardness. Choosing a much higher Tg than necessary for this purpose tends to decrease the toughness of the material. Formulating near a 1:1 stoichiometry of amine-hydrogen to epoxy groups, and sufficient baking or curing, also helps ensure that the maximum obtainable Tg is achieved, while producing the maximum number of bonds, thus contributing to the strength. Although formulating away from a 1:1 stoichiometry will depress the Tg and provide more flexibility, as long as the Tg is not too high to start with, it also leaves unreacted groups in the system that can destabilize it over time. As an example, unreacted epoxy groups can hydrolyze over time, leading to increased moisture sorption and related thermo-mechanical changes.
As the Tg of the cured resin increases, the toughness and toughenability of the polymer declines, as does the strain at break, or percent elongation. When aromatic amines or cycloaliphatic amines are used to fully cure a DGEBA type resin, the resulting Tgs (ranging from about 155 degrees C to >210 degrees C) are so high above most ambient use temperatures that toughness is low and only brittle failures occur. By blending in some percentage of amine hardener that provides a lower Tg, while accounting for stoichiometry, some toughness can be recovered. At the same time, one can significantly vary the working-time, or pot-life, of the new system by choosing faster- or slower-reacting aliphatic amines.
Modifying the modulus of thermosetting epoxy systems may be done in a variety of ways. If the modulus is a bit low because the Tg range of the polymer overlaps the use temperature a bit, the modulus may be raised by formulating to increase the Tg somewhat. As long as the Tg range is sufficiently higher than the use temperature, further crosslinking actually decreases somewhat the modulus of the glassy polymer. As might be expected then, it has been observed that as one moves away from a 1:1 stoichiometry, or from full curing, the modulus will increase, at least until the Tg is depressed to the point where the Tg range encroaches upon the service temperature and this effect in dropping the modulus becomes dominant. Increasing the modulus by either undercuring or formulating away from a 1:1 stoichiometry leaves unreacted groups in the polymer, which may affect durability.
Hardness should typically show a positive correlation with modulus, except in cases where the hardness measurement is done at a surface that does not represent the bulk material. This can happen, for example, when an indenter-type device (e.g., Durometer) is pressed into a surface exhibiting amine-blush related tackiness.
Obviously, it is impossible to maximize simultaneously several of the thermal and mechanical properties, thus good formulation may often amount to managing trade-offs of the most desirable properties (Figure 2).
Environmental-Related Performance
Polymer or material changes caused by the service environment may lead to premature failure. Examples of such polymer changes and service life conditions include: thermal degradation; thermo-oxidation; photo-oxidation (e.g. from sunlight/UV-light); cyclic fatigue (e.g. from vibration); physical aging (densification); erosion; ESCR (Environmental Stress-Cracking Resistance); and sorption of water, solvents, etc. The effect of different service life conditions may be synergistic, leading to surprisingly quick failures. Polymer performance in these areas may be related to both the degree of crosslinking and the chemical nature or polarity of the amorphous polymer.
For example, sorption of a liquid by the cured epoxy may lead to chemical changes as well as mechanical changes. For fully cured epoxy resins, liquid sorption has led to failures, often interrelated, from: swelling, modulus loss, strength loss, stress cracking (ESCR), weight gain, gloss loss, hardness loss, adhesion loss and coloration. For this reason, comparative testing of different amine hardeners in a given service environment may be of crucial importance in selecting the best amine hardener or blend.
Amines as Modifiers
Polyetheramines (PEAs) exhibiting reduced reactivity due to steric hindrance near the amine (e.g. with JEFFAMINE® D-230 amine) have been used in amine blend hardeners to simultaneously reduce the viscosity and reactivity of polyamides, Mannich bases, and other high-viscosity amines. This can broaden their application.
Cycloaliphatic amines, ethyleneamines (e.g. DETA, TETA, and TEPA) and unhindered polyetheramines may be blended with standard polyetheramines to increase their reactivity. Cycloaliphatic amines and ethyleneamines may also be blended into polyetheramines in applications where a higher Tg is needed to ensure good hardness at somewhat elevated service temperatures.
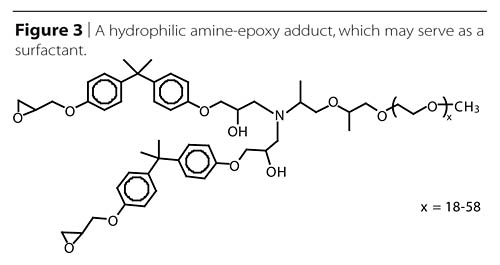
Adduction of hydrophilic polyetheramines has been used to create reactive surfactants, thus allowing reduction or elimination of un-bound surfactant additives, which may migrate over time and change performance (Figure 3). Reaction of a few percent of a hydrophilic amine with an excess of epoxy resin has been shown by Klein and Darragas2 to provide water dispersibility to the entire mixture.
Also, hydroxyethylamines have long been used to modify epoxy resins for preparation of electrodeposited coatings used as automobile body primers.
Amine Blush and Amine Storage Considerations
The topic of amine "blush" formation (formation of ammonium carbamates by reaction with atmospheric carbon dioxide) has been discussed broadly elsewhere3 but it seems useful to mention it briefly here, too. Nearly all amine hardeners appear to readily absorb atmospheric carbon dioxide. This poses a variety of possible problems for the epoxy formulation, and steps should be taken in storage and handling to avoid repeated air exposure of the amine hardeners prior to curing.
In epoxy coating applications, carbon dioxide reacting with amine hardener at the surface may cause low hardness, poor paintability, poor intercoat adhesion, water-spotting, poor solvent resistance and poor gloss retention. In molding operations, amines contaminated by ammonium carbamate formation can release carbon dioxide at elevated temperatures, causing large pressure build-ups and spraying. In thick parts or castings, a similar effect may occur where bubbles or foam is generated in the center sections, where the highest temperatures are reached due to the reaction exotherm.
For those amine hardeners in which the ammonium carbamate reaction product is insoluble, carbon dioxide sorption typically causes white solids formation, which in some processes leads to poor mixing, line and orifice plugging, and surface defects. For amines, such as polyetheramines, in which the ammonium carbamate remains soluble, higher viscosity may be seen as more and more carbon dioxide is sorbed.
Summary
Use of amine compounds in epoxy resin curing is a primary way by which the use of epoxy resins has greatly expanded in the decades since their commercialization. Even greater versatility is being made available to the epoxy formulator as new hardeners are developed to meet unusual processing and performance requirements. Creative use of amine blends can provide a wide range of processing, thermal and mechanical performance combinations.
JEFFAMINE® is a registered trademark of Huntsman Corporation or an affiliate thereof in one or more, but not all, countries. VERSAMID® and GENAMID® are trademarks of Cognis Corp.
Definitions
Crosslinking: A bridging of polymer chains that creates a polymer network. Once crosslinked, the polymer will no longer flow upon heating and is termed a thermoset or thermosetting polymer or plastic. Thus crosslinking, a characteristic of most polymerized epoxy resins, is responsible for the final thermal and mechanical performance of the material.
Curing (also called crosslinking) : The term also may apply to the earlier stages of polymerization wherein fluids increase in viscosity prior to gellation and hardening.
Epoxy, epoxide, oxirane: A three-member cyclic ether that serves as the primary reactive functional group in most lower-molecular-weight epoxy systems. Higher-molecular epoxy resins may contain hydroxyl (OH) groups that may serve as reactive groups in addition to or in place of epoxy groups.
Exotherm: A spontaneous evolution of heat caused by a chemical reaction, such as the curing of an epoxy resin formulation. This typically leads to an increase in temperature, which can sometimes lead to thermal degradation.
Gelation: The point in the polymerization reaction at which a polymer network just begins to form as a result of crosslinking. At the gel point, polymers just begin to show resilience or elasticity.
Gel Time: The time elapsed between mixing the hardener into the epoxy resin and when the mixture just starts to gel and form a crosslinked polymer network.
Glass Transition Temperature (Tg) : The Tg of a polymer is usually defined as the mid-point of the temperature range over which an amorphous (glassy, non-crystalline) plastic goes from being relatively hard and rigid to being relatively flexible (i.e., from glassy to rubbery). The mid-point of this range is somewhat dependent upon the method and rate of measurement.
Hardener, Curing Agent,
Co-reactant: These terms are often used interchangeably to describe compounds that polymerize or co-polymerize with epoxy resins to produce usable materials. The polymerizing resin becomes harder than the starting material, thus the name hardener.
Pot-life, Working Life (Time) : The time available for application of the epoxy formulation. The end of pot-life is application-dependent and may occur, for example, when the mixture gels or when its viscosity exceeds the viscosity at which it can be properly mixed or applied.
Stoichiometry: The number relationship between reactive groups in a reaction. Because unreacted groups can lead to property changes over time, a one-to-one ratio of epoxy groups to amine-hydrogen groups is typically desirable, though not always necessary, in epoxy formulations.
Polyetheramines 25 - 100 degrees C Tg
Moderate Tg, thus flexible
Long pot-life
Slow curing, low exotherm
Only slight odor
Very low color
Low viscosity
Ethyleneamines and their Adducts
110 - 130 degrees C Tg
Somewhat brittle
Short pot-life
Fast curing, high exotherm
Handling concerns/odor
Low viscosity
Polyamides and Amidoamines
40 - 100 degrees C Tg
Moderate pot-life
Fast curing, high exotherm
Colored
Very good corrosion resistance
Handling concerns/odor
Higher viscosity
Arylyl Diamines
Somewhat brittle when fully cured
Fast curing
Low temperature curing
Chemical and water resistance
Cycloaliphatic Amines
145 - 175 degrees C Tg (baked cure)
Somewhat brittle when fully cured
Intermediate pot-life
Moderate exotherm
Some odor
Very low color
Often low viscosity
Aromatic Amines
Higher Tg, 160 - 220 degree C (baked cure)
Somewhat brittle
Slow curing, higher temperatures
High strength
Colored
Toxicity concerns
High viscosity or solid at room temperature
Mannich Bases and Phenalkamines
50 - 55 degres C Tg (ambient cure)
Somewhat brittle
Curing as low as freezing temperatures
Colored
Chemical resistance
High viscosity

Report Abusive Comment