

Nonetheless, the polyol and isocyanate pre-polymer manufacturers recognized the potential of this technology and have been persistent in their efforts to provide meaningful information that confirms the viability of 2K waterborne polyurethanes. To date, their efforts have been successful in generating a high level of interest due to well-planned studies exhibiting 2K waterborne polyurethanes with performance comparable to conventional 2K solventborne urethanes. In addition, there have been some significant commercial successes. According to Melchoirs et.al., two-component waterborne urethane coatings developed by Bayer have demonstrated comparable performance to their solvent-based counterparts in IM applications, auto refinishing and in special-effects systems, such as "soft-feel" coatings.1 Other suppliers have also developed very viable technologies utilizing various types of polyols, which have proven to rival their solventborne counterparts in virtually all aspects of performance in IM, wood, automotive and aerospace applications.2 However, the growth of this technology remains slower than expected, despite the excellent performance and extremely low-VOC and HAPs-free formulations offered by many of the existing technologies.
While many will speculate that this slow acceptance of 2K waterborne polyurethane technology is due to the industry acceptance of high-solids 2K urethanes to meet current and future VOC regulations, there are many cases wherein high-solids systems are simply not well suited to the application. There are numerous applications ranging from wood furniture and wood flooring to automotive refinish and aerospace coatings where the slow speed of dry and short pot life of most high-solids urethanes limits the practical usage of these systems.
In some cases, the relatively high RMC of these polyol-isocyanate combinations is cited as a limiting factor in the commercial success of these systems. These cost considerations are largely due to the high cost of the waterborne polyols and their companion polyisocyanates. Much of the presently available polyol technology involves the use of relatively low-solids polyacrylic dispersions and polyurethane dispersions, while the polyisocyanate is often a hydrophilically modified isocyanate prepolymer. Both are considerably more expensive than the acrylic polyols, polyester polyols and aliphatic isocyanates typically utilized in conventional solids, two-package solventborne urethanes.
Figure 1 exhibits a baseline example of the impact that higher polyol and isocyanate cost has on the coating RMC. This graph depicts the relative polyol and isocyanate costs in a conventional solids, solventborne polyester-isocyanate system formulated at a 1.05:1 NCO to OH ratio, on equivalents. In addition, the graph also includes the polyol and isocyanate costs for two commercially available waterborne polyol-isocyanate systems. The first waterborne system is a polyester-polyurethane dispersion cured with an HDI trimer at the recommended stoichiometry of 1.5:1 NCO to OH. The second is a waterborne acrylic emulsion designed to be crosslinked with a hydrophilically modified isocyanate, also at a NCO to OH ratio of 1.5:1 on equivalents. As this figure clearly demonstrates, the increase in polyol and isocyanate costs can equate to as much as $3.00 - $4.00 per gallon on polyol and crosslinker costs alone.
This article introduces a novel water-reducible polyester polyol, which allows for the formulation of 2K waterborne urethanes that are competitive with their solventborne counterparts in both performance and RMC, and shows how this technology responds to various aspects of formulation including: polyisocyanate selection, stoichiometry, catalysis and formulating latitude.

The Technology
In recent years, U.S. Polymers, Inc., St. Louis, MO, has been actively pursuing the development of a low-cost, water-reducible polyester polyol for 2K waterborne urethanes. The intent was to produce a product line for formulating coatings with the balance of cost and performance competitive with conventional and high-solids systems. The latest product to achieve commercial status in this line of products, W2K®2002, is a 90% NV waterborne polyester polyol designed specifically for use in two-component waterborne urethane coating systems. This waterborne polyol is somewhat lower in molecular weight compared to many acrylic emulsions or dispersions, thus allowing for its high-solids supply form. In addition, this polyol has a higher degree of hydroxyl functionality, thus enabling the formulator to achieve a crosslink density more comparable to those of solventborne systems in very low-VOC, waterborne alternatives. In addition, the unique emulsification mechanism employed in W2K2002 polyester polyol allows for rapid emulsification of conventional 100% NV aliphatic isocyanates, thus eliminating the increased cost imparted by the use of hydrophilically modified isocyanates required for use with other polyol technologies. Polyol cost is also quite favorable. Figure 2 demonstrates the relative cost of several waterborne polyols and a solventborne polyester standard on a dry pound basis and the cost-effectiveness of this new water-reducible polyester polyol technology.
Polyisocyanate Selection
While the development of waterborne polyols for 2K waterborne urethanes has been underway for many years, this aspect of technology remains in the early stages of development. To date, there is no preferred method for rendering the polyol water dispersible and, as time has shown, it is this aspect of the dispersion that basically determines the type of isocyanate one must utilize with a given waterborne polyol.In general, many of the waterborne hydroxyl functional acrylic and polyurethanes dispersions designed for use with isocyanates are not extremely efficient in the emulsification of conventional, 100% NV isocyanate. As a result, these polyols are far more compatible with hydrophilically modified isocyanates. Conversely, there are waterborne polyols that utilize water-soluble or water-reducible technologies that are generally quite efficient in emulsifying the conventional 100% NV isocyanates. As a result, there has been much discussion over the issue of polyisocyanate selection and its ultimate impact on coating performance.
The type of polyisocyanate most commonly used in the formulation of all two-component waterborne urethanes is a HDI trimer. However, how this isocyanate is emulsified varies from polyol to polyol, depending on how the polymer is rendered water miscible. Early studies conducted by many of the early pioneers of 2K waterborne urethanes indicated that waterborne polyols, or more specifically acrylic and polyurethane dispersions, could not emulsify conventional, hydrophobic isocyanates sufficiently to allow for good film formation and performance. Most often, problems with stability, gloss and general film aesthetics were reported, along with some loss of crosslink density and performance. Prior studies conducted by Melchoir and others concluded that one must employ the use of a water-miscible isocyanate in order to produce a stable 2K waterborne urethane coating. If conventional isocyanates are used, the main factor in ensuring the good dispersibility of the hardener in the binder is low viscosity. In the case of higher-viscosity polyisocyanates, the droplets remain relatively large. The dispersion is unstable, and the polyisocyanate settles.3 However, others have reported that the current cost of hydrophilically modified isocyanates adds greatly to the formulated cost of the applied coating and can also have a negative effect on water and acid resistance of the cured film.
In response to these limitations and patent considerations, many polyol manufacturers have undertaken the challenge of simply developing a waterborne polyol that has sufficient emulsification strength to properly emulsify conventional aliphatic isocyanates, thus producing stable coatings with good film-formation properties. This technique has been employed successfully in the development of 2K waterborne epoxy coatings for well over 30 years. In these systems, an amine functional curative was designed to fully emulsify the liquid epoxy resin. It is speculated that through this method one can achieve the greatest degree of polyol-crosslinker interaction, thus improving the coating compatibility and stability. In addition, this approach is said to increase the probability of both the polyol and the crosslinker being in the same phase, as opposed to having two materials that are both water miscible and compatible in blends, but are limited in particle interaction except when the blend is prepared under very high-shear mixing conditions.
Studies reported by Bassner and Hegedus have shown that it is possible to utilize this approach in developing waterborne polyols that possess the necessary emulsification strength to adequately emulsify conventional aliphatic isocyanates.4 Based on their findings, it was concluded that this ability to emulsify non-aqueous crosslinkers decreases film-formation issues surrounding particle coalescence and ultimately improves coating performance.
With this in mind, the W2K2002 waterborne polyester polyol was developed to allow for the use of conventional isocyanate technology and to provide a balance of cost and performance comparable to conventional 2K polyester-isocyanate systems. In this design, several factors were of primary consideration. These factors included the development of an efficient emulsification package that would circumvent known limitations common to existing 2K waterborne urethane technologies, including isocyanate selection and formulating latitude. The W2K2002 features a dual mode of emulsification wherein the use of emulsifier, in conjunction with a carefully controlled level of carboxylic acid functionality, allows for reduction with water and the ability to emulsify conventional polyisocyanates and other resinous modifiers. The use of this unique mode of emulsification eliminates the water sensitivity characteristic of many polyols stabilized with emulsifiers/surfactants and minimizes the out-gassing and formation of excess urea often encountered with systems rendered water dispersible solely through acid functional-amine neutralization.
In addition, the degree of emulsification strength and the resulting coating stability of this novel polyester polyol can be altered through the addition of very low levels of ammonium hydroxide. Studies have shown that levels as low as 0.5 % on polyol solids can have a significant impact on stability and coating aesthetics. However, higher levels of ammonium hydroxide can be used judiciously to produce additional water miscibility and emulsification strength to accommodate modifications with other polyols. This, in turn, provides the coatings formulator with a greater range of latitude to develop a system meeting the needs of the market at a very competitive RMC.
Effects of Catalysis
Traditionally, 2K solventborne urethane coatings have been catalyzed to promote more rapid polyol-isocyanate reaction to ensure that the applied coating exhibits the desired speed of dry and cure. While it is true that much of the anticipated reaction would occur without catalysis, full cure of the coating can be retarded by as much as 3-4 weeks. With catalysis, adequate cure to allow for field service can be achieved in as little as 48-72 hours. There are a variety of catalyst types that effectively promote this reaction, but those generally favored by formulators are tin-based compounds such as dibutyltin dilaurate (DBTDL). This catalyst generally offers an excellent balance of usable pot life and cure speed and is the most widely used catalyst in the formulation of 2K urethane coating systems.However, many of the commercially available waterborne polyols do not recommend the use of any catalyst, despite the potential attributes, due to the problems encountered with maintaining an acceptable pot life due to premature out-gassing or excessively rapid reaction. It is well documented that 2K waterborne urethane systems are among the most difficult waterborne technologies to develop and formulate. This is due to the variety of competing reactions, in addition to the preferred polyol-isocyanate reaction, which can occur once the two components have been blended.
In most cases, the most limiting of these potential isocyanate side reactions is that which occurs with water in the coating system. While water contamination has long been recognized as a potential problem in achieving the expected properties in a 2K solventborne coating, in 2K waterborne urethanes one must now deal with this same reaction, with water now composing as much as 50-60% of the total coating volume. Fortunately, previous studies conducted with other waterborne urethanes have shown that the reaction of isocyanate with the hydroxyl groups of the polyol begins almost immediately after blending of the two components.5 In addition, prior studies have also shown that the isocyanate-water reaction generally does not occur, in abundance, for more than two hours after blending.
Unfortunately, this alone does not alleviate the potential problem, particularly when the coating is catalyzed. Most catalysts are not selective in their activity and thus catalyze the isocyanate-water reaction in addition to the polyol-NCO reaction. This has been found to be quite common with tin-based catalysts such as DBTDL. While there are other catalysts that are said to be more selective in their activity, such as recently introduced zirconium complexes, most fail to produce the high level of catalytic activity achieved with DBTDL, and their activity is limited in certain 2K waterborne urethane technologies.
In contrast to many other waterborne polyols, the unique emulsification mechanism employed in the preparation of W2K2002 waterborne polyester polyol does more than emulsify conventional isocyanates. This polyol also seems to encapsulate or wrap around the isocyanate, thus reducing easy accessibility of water to the NCO functionality and providing for intimate contact between the polyol and isocyanate. This, in turn, allows for the use of conventional catalysis and the latitude in formulation to change catalyst to achieve the desired pot life and cure rate, much like one would do in a conventional solventborne 2K urethane. To demonstrate the latitude of this response, a study was conducted wherein a 16% PVC gloss white enamel (based on the W2K2002 crosslinked with the trimer of HDI at an NCO to OH stoichiometry of 1.5:1) was catalyzed with four tin-based catalysts with varying degrees of catalytic activity. The catalysts evaluated included Metacure T-1 (dibutyltin diacetate), Metacure T-12 (dibutyltin dilaurate), Metacure T-120 (butyltin mercaptide) and Metacure T-9 (stannous octoate). The relative order of the typical catalytic activity for these catalysts in 2K urethanes is as follows: dibutyltin diacetate > dibutyltin dilaurate > butyltin mercaptide > stannous octoate.
Table 1 demonstrates the effects of catalysis on usable pot life, dry times, hardness development and gloss using DBTDL, butyltin mercaptide, and stannous octoate at levels of 0.0125%, 0.0125 % and 0.06 % on total resin solids (TRS), respectively. (Note: the effects of dibutyltin diacetate are not shown in this Table due to its extreme catalytic effect and resulting limitations on the coating pot life.)
Of the catalysts evaluated, the butyltin mercaptide at a dosage of 0.0125 % on TRS offers an excellent balance of pot life and cure rate, coupled with good aesthetics and an excellent balance of physical properties. However, more importantly, the performance trends of catalysts in W2K2002-based enamels correlate well with that observed in their solventborne counterparts, thus providing the formulating latitude necessary to adapt this polyol technology to meet the requirements of many facets of the urethane coatings market.
Stoichiometry
In the formulation of conventional and high-solids urethane systems, most coatings employ a NCO to OH stoichiometry of 1.05:1 equivalents based on TRS. The slight excess of isocyanate assures complete reaction of all hydroxyl groups, with any residual isocyanate ultimately reacting to form polyurea. In the case of 2K waterborne urethane systems, some of the polyisocyanate groups are consumed in reactions with water, yielding carbamic acid that immediately decomposes to liberate carbon dioxide and an amine. The amine by-product can then react with other polyisocyanate groups to form polyurea. Often the polyurea formation is irrelevant given that excessive out-gassing due to the carbon dioxide formation will often mark the end of the usable pot life of a 2K waterborne urethane coating system.As a result, it has become customary to utilize a significant excess of polyisocyanate to compensate for these potential interactions. A typical NCO to OH stoichiometry for a waterborne urethane is 1.5:1. However, moderate increases in NCO indexing can yield significant changes in coating properties. In 2K waterborne urethanes, NCO indexing recommendations for some polyols are as high as 4:1, which obviously results in a significant increase in RMC, particularly if a hydrophilically modified polyisocyanate is required.
Sometimes the dry film thickness achievable in 2K waterborne urethane coatings is limited to ~ 2 mils by out-gassing that results from CO2 generation in the coating. This can be a problem in actual applications since a modest increase in film thickness can result in very poor film appearance due to entrapped air. This can be even more relevant in systems with the high levels of excess NCO characteristic of many technologies. While some CO2 liberation is inevitable, it is possible to retard the CO2 formation through polymer design and formulation. Studies conducted with the W2K2002 water-reducible polyester polyol have shown that an NCO index as high as 1.5 to 2.0 can be accommodated without early out-gassing, given proper catalyst selection and dosage level. At a stoichiometry of 1.5:1, W2K2002-based formulations provide excellent overall performance and a usable pot life of 4-6 hours. The end of the pot life is marked by a gradual increase in out-gassing. However, this CO2 release is latent in nature due to the ability of the W2K2002 to colloidally protect the isocyanate from premature reaction with water and the proper catalyst selection. As a result, gloss enamels based on this polyol can be applied at dry film thickness up to 3.5 mils without any of the film imperfections often observed in 2K waterborne urethane systems. The same has been found to be true of this system formulated with a 2:1 NCO to OH stoichiometry, although out-gassing does appear more readily due to the higher isocyanate content. This NCO increase will further enhance properties such as hardness development, solvent resistance, and resistance to numerous fluids including brake fluid and Skydrol hydraulic fluids, but with a reduction in usable pot life to 2-3 hours. NCO indexes higher than 2:1 are not typically recommended with this polyol due to expected decreases in usable pot life, gloss, and early hardness development.
Cost-Effective Performance
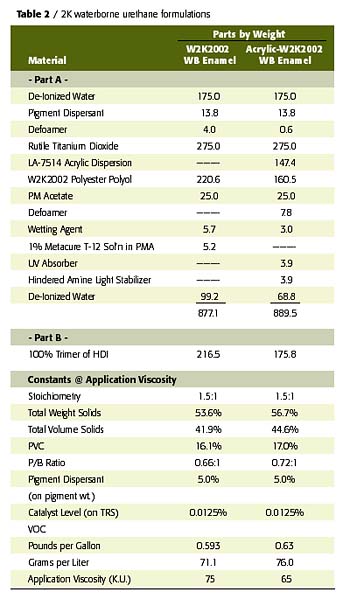
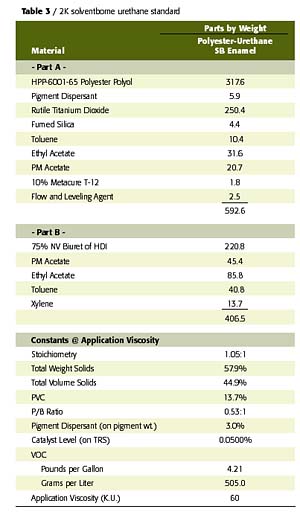
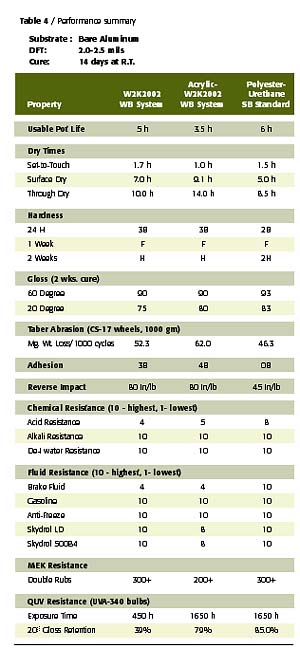
To confirm the performance potential of this low-cost waterborne polyester polyol, a comparative study was conducted wherein two W2K2002-based gloss white enamels were evaluated in a side-by-side comparison with a conventional solids, 2K solventborne polyester-urethane. The first waterborne coating was an unmodified, W2K2002-based enamel and the second was an acrylic-modified, W2K2002-based enamel (70% polyester-30% acrylic on solids) with a UVA/HALS package. The solventborne polyester resin was a 65% NV polyol in EE acetate that utilizes the biuret of HDI for crosslinking. The acrylic modification to the W2K2002 imparts desirable improvements in the performance of this polyester in areas such as speed of dry and UV durability at levels as low as 10% of total polyol solids. The formulation compositions and physical constants of the prepared coatings are listed in Tables 2 and 3.All coatings were applied to bare aluminum via conventional air spray to a dry film thickness of 2.5-3.0 mils. Each coated panel was then allowed to cure at room temperature for a period of 14 days, prior to any testing other than periodic hardness and gloss measurements. All cured films were then tested for physical, chemical and accelerated exposure properties using ASTM test methods, where applicable.
In review of the coating performance demonstrated in this study, focus was placed on those properties that are key to the expected performance of solventborne urethanes. These properties include film aesthetics, speed of dry, hardness, mar and scratch resistance, flexibility, adhesion, abrasion resistance, UV durability, and resistance to solvents and corrosive hydraulic fluids, such as brake fluid and Skydrol.
These properties are often the most important in the interior and exterior use of 2K urethane coating systems in IM, automotive refinish and aerospace applications. As indicated by the data in Table 4, the W2K2002 waterborne 2K urethane enamels exhibit performance comparable to the solventborne standard in nearly all of these key aspects of performance. In fact, the waterborne 2K urethane systems exhibited superior performance in several aspects of testing, including adhesion and flexibility. Further study has confirmed this trend is consistent with application over a variety of ferrous substrates as well.
Due to the low inherent molecular weight and Tg of this polyol, the unmodified W2K2002 set-to-touch times are somewhat slow relative to the solventborne standard and the acrylic-modified WB system. However, dry through times and hardness developments are quite comparable, despite the lower catalyst level employed in the 2K waterborne system. The acrylic modification of this polyester polyol system provides the early dry typical of the conventional solids, solventborne standard due to the high Tg of the acrylic resin. The notable improvement in UV durability in the acrylic-modified WB system is credited to the use of the UV absorber, HALS package, in addition to the contribution of the acrylic resin
As expected, all coatings exhibited very good crosslink density as indicated by the hardness, abrasion resistance, and solvent and fluid resistance of these coatings. The acrylic modification does detract from abrasion and solvent resistance of the cured films. However, this could be a reasonable compromise for some applications given the improved dry times and UV durability achieved with this modification.
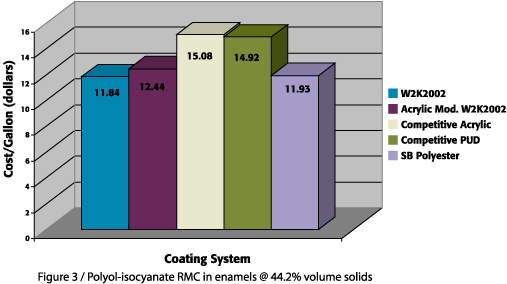
However, despite the comparable performance of the waterborne 2K urethane systems, the viability of this technology will always come back to cost. While most coating manufacturers recognize the cost of research and development and its contribution to the cost of new technology, the cost of many of the existing waterborne technologies is simply deemed unacceptable for most applications. However, the 2K waterborne urethane systems based on the W2K2002 water-reducible polyol are quite comparable to the conventional solids, solventborne urethane standard in cost, due to the low cost of the polyol and the ability to utilize conventional polyisocyanates. The relative polyol and isocyanate costs of these coating formulations are displayed in Figure 3. These costs are based solely on polyol and isocyanate cost in formulations all prepared at 44% solids by volume. As shown, the cost of these unique 2K waterborne urethane coatings is within 5% of the solventborne standard, as compared to the 15-20% cost differential of other polyol technologies. Given this cost-performance balance, it now appears possible to formulate waterborne 2K urethanes that rival their solventborne counterparts in both performance and formulated cost.
Formulating Latitude
In addition to allowing for the emulsification of conventional isocyanates, the W2K2002 water-reducible polyol also offers several other capabilities, which allow for greater latitude in formulation. The expected response of this polyol to stoichiometric and catalytic changes allows for the design of a coating with specific performance strengths, similar to those observed in solventborne technologies. The feasibility of producing high-performance 2K waterborne urethane coatings using this technology is further enhanced by the high degree of compatibility this polyol has exhibited with other vehicles.The emulsification strength of this technology also contributes to its potential use as a modifier for other waterborne polyols. In this capacity, this polyol can enhance the emulsification strength of the primary polyol to allow for the replacement of hydrophilically modified isocyanates with conventional isocyanates. Additional capabilities of this polyol as a modifier include the following:
- The ability to improve the crosslink density of less-functional waterborne polyols;
- Use of this polyol as a grinding vehicle for emulsion and dispersion polyol technologies, thus allowing for a 60-80% reduction in pigment dispersant;
- Improved color acceptance;
- The opportunity to reduce cost by replacing a portion of more expensive waterborne polyols.
Summary and Conclusions
The purpose of the studies presented was to demonstrate the potential of producing a line of 2K waterborne urethane coatings products that can compete, in new and existing markets, with conventional and high-solids systems, with an RMC comparable to that of the solventborne standards. As noted, these waterborne urethane coatings are often near zero in VOC, and the absence of these volatile organics could well create new opportunities for the use of two-component urethane technology, including a variety of new applications due to the less-aggressive nature of these systems. Of particular interest are plastic applications, such as automotive or business machines, and numerous general industrial-finishing applications.In these studies, it has been demonstrated that much of this potential is based on polyol selection and the emulsification strength of this polyol. Through polyol selection it is possible to eliminate the need for costly, hydrophilically modified isocyanate crosslinkers, while maintaining a performance level quite comparable to that of conventional and high-solids coatings, with the net result being a cost savings of 15-20%. The subject polyol also provides the desired response to stoichiometric changes and catalysis, thus adding to the formulating latitude often needed to optimize products for a given application. Additional formulation latitude was demonstrated through modification of this waterborne polyol with an acrylic dispersion resulting in significant improvements in speed of dry and UV durability, while maintaining very good overall coating performance in other facets of testing.
For more information on U.S. Polymers, Inc., contact 300 E. Primm Street, St. Louis, MO 63111; phone 314/638.1632; fax 314/638.3100; or e-mail uspolymr@aol.com.
For more information on Specialty Coating Services, Inc., contact 8003 Vine Crest Avenue, Unit 2, Louisville, KY 40222; phone 502/327.7252; fax 502/327.8848; e-mail mikej@scslabs.com; or visit www.scslabs.com.
References
1 Melchoirs, M.; Kobusch, C.; Noble, K.; and Sonnatag, M. "Aqueous Two-Component Polyurethane (2K-PUR) Coatings: An Evolving Technology", Presented at the International Waterborne, High Solids, and Powder Coatings Symposium, New Orleans, LA, February 10, 1999.
2 Jacobs, P.; Best, K.; Dvorchak, M.; Shaffer, M.; Wayt, T.; and Yu, P. Two-Component Polyurethane Coatings: Now and Into the Next Century, PCI, 128, October 1998.
3 Melchoirs, M.; Kobuswch, C.; Noble, K.; and Sonnatag, M. "Aqueous Two-Component Polyurethane (2K-PUR) Coatings: An Evolving Technology", Presented at the International Waterborne, High Solids, and Powder Coatings Symposium, New Orleans, LA, February 10, 1999.
4 Bassner, S.L; and Hegedus, C.R. A Review of Two-Component Water-Borne Polyurethane Coatings for Industrial Applications, JPCL, 64, September 1996.
Hegedus, C.R.; Gilicinski, A.G.; and Haney, R.J. Film Formation Mechanism of Two-Component Waterborne Polyurethane Coatings, J. Ctgs. Tech., 68, No. 852, 57, (1996).

Report Abusive Comment