In the European market, i.e., within the enlarged EU’s 25 countries, almost exactly 200,000 t of anti-corrosion coatings valued at €640 million were consumed in 2004.1This figure includes all building and maintenance activities in the construction sector, i.e., structural and hydraulic engineering (including pipelines but excluding marine/offshore). About half of this volume was attributable to new construction (approximately 8 million tons of steel with roughly 160 million m2 of coated surface area), of which half again (50,000 t) was consumed directly by the steel or OEM manufacturers. The remaining portion of new coatings was applied by contract coaters or applicators at a construction site. The application of refurbishment coatings (~100,000 t) took place almost exclusively at construction sites. Light-duty corrosion protection, or what is known as “general industrial coating,” is only reflected in these figures insofar as it involves coatings that cure at room temperature (e.g., heavy, agricultural and construction machinery).

Success Factors of Anti-Corrosion Coatings
Raw material manufacturers and coatings producers are working together on concepts for innovative anti-corrosion coatings. Developments in the industrialized nations of the EU are currently driven by an effort to obtain efficient solutions in view of the changing quality and environmental demands imposed on corrosion protection.
With regard to the efficiency of a coating, a distinction must be made between the construction phase and the subsequent period of continuous use. The success factors shown in Table 1 must be taken into account in the construction phase.
The parameters shown in Table 2 affect efficiency during long-term use. The aim is to achieve high durability as a function of the type of structure involved.

The complex fields of application result in a variety of different requirements for anti-corrosion coatings. To address this complexity, standards for the main applications have been established on the European and national levels that define minimum coating requirements. Most of these standards also characterize the coatings by binder type to reflect the “state of the art” (e.g., Part 5 of ISO 12944 for corrosion protection in structural engineering).2
Laboratory corrosion tests for coatings are used in ISO 12944 – Part 6 to define quality characteristics. The tests are intended to simulate practical requirements as realistically as possible in a short period of time. Specific regulations issued by national agencies also exist, such as Germany’s Terms and Conditions of Technical Supply and Testing (TL/TP - Technischen Liefer- und Prüfbedingungen) issued by the German Federal Highway Research Institute (BAST – Bundesanstalt für Straßenwesen),3and internal specifications issued by major industrial corporations (e.g., Bayer Company Standard for Corrosion Protection),4which are intended to ensure the greatest possible correlation between the test procedures described and the conditions prevailing for a structure in practice.

As a result of the European VOC Directive and generally increased awareness, environmental compatibility has for some years been the focus of development in corrosion protection. Anti-corrosion coatings make up about 4% of all coatings used in Europe and contribute some 100 kt to the EU-wide total of 3 million t solvent emissions in the entire coatings sector.5,8In a representative corrosion protection study conducted recently by CEPE among members active in corrosion protection, which reflected about 2/3 of the market (87 million l), the figures (Table 3) emerged for the VOC content of coating systems currently sold in Europe.
These figures do not include anti-corrosion baking coating systems used in general-purpose industrial coating applications. If they were added, the percentage of water-reducible 1K and 2K systems with a total VOC content between 60 and 80 g/l would be in the region of 25%.
The EU Directives and their national Implementing Regulations are relevant for limiting the solvent content by law. They distinguish between outdoor applications and applications in stationary installations (Tables 4 and 5).
For four years now, Swiss regulations on solvents have required manufacturers and suppliers to pay a tax on the solvent contained in a coating (amounting to 3 SFR/kg as of this year). Consequently, there are already good market opportunities in Switzerland for coating systems with the lowest possible VOC content. The VOC issue will not be put to rest in Europe with the new VOC Directive, because successor models are already under discussion.
One such model is the Clean Air for Europe (CAFE) program initiated by the EU Environmental Authority, which is to achieve even greater sustainability by 2020. The concept, proposed by a group of experts, is a simple regulation that starts with the manufacturer, introduces product-specific solvent limits and is to be mandatory for all installations and structures (even those with <5 t/year solvent emissions). The aim is to generally reduce emissions caused by anti-corrosion coatings to <27 g VOC/100 g coating (the limit value in the EU VOC Directive is currently restricted to large installations).8As a result, the goal of developing modern systems in Europe is to achieve the lowest possible VOC content coupled with optimal efficiency compared to other systems.

System Comparison: High-Solids Topcoats
Of the 72,000 total tons of 1K and 2K topcoats consumed in the EU in 2004, an estimated one-fourth were used for OEM coatings in stationary installations. Therefore, the relevant market for topcoats affected by the VOC Directive is estimated at about 20,000 t with a material value of about €100 million.The current 2K polyurethane topcoat (as per TL/TP KOR, Sheet 87) can be taken as the standard against which newly developed, modern high-solids products can be measured. The workability (three-hour pot life, completely dry in eight hours in a standard climate, spray/brush/roller application) and the processing reliability (thorough curing under various climate conditions, sufficient overcoat thickness tolerance) have set the standard in practice for over 20 years. Innovative activities for modern systems are mainly driven by the desire for a lower solvent content (standard 2K polyurethane topcoats currently on the market contain about 420 – 550 g/l VOC) and a rising demand for efficiency (ideal goal: to cut the drying rate by half while maintaining the same pot life). Typically, hydroxy-polyacrylates with 60% solids and 2.6% OH content (referred to 100% solids) and aliphatic biuret-polyisocyanates with 75% solids and 22% NCO content are used as binders in conventional systems.
Recently, three developments have been competing in the topcoat sector of heavy-duty corrosion protection:
- 2K polyurethane: high-solids OH-polyacrylate cured with aliphatic polyisocyanate;
- 2K polyurea: NH-polyaspartics cured with aliphatic polyisocyanate; and
- 2K polysiloxane: EP/polysiloxane hybrid cured with aliphatic amine.
High-Solids Polyurethane
The 2K polyurethane topcoats used in corrosion protection essentially are based on hydroxy-polyacrylates reacted with HDI-polyisocyanates (biuret or trimer). High-solids polyurethanes differ from conventional systems in terms of the modified composition of acrylate monomers used in production. Polyester-polyols are also used to a lesser extent for coatings with greater resistance to chemicals.If a coating is to cure in a standard climate after a maximum of 10 hours, a sprayable formulation with a VOC content of up to about 340 g/l can be achieved with modern high-solids OH-polyacrylates. For brush-on and roll-on formulations, the lower limit of the VOC content under identical conditions is 300 g/l. In most cases, however, a reduced pot life of roughly 1.5 hours and a somewhat lower coating hardness compared to the standard must be accepted in return (pendulum hardness after 7 days in storage at room temperature is 50-70 s compared to 90 s with the conventional standard).
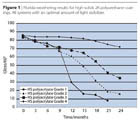
Modern high-solids hydroxy-polyacrylates differ with regard to their OH content and monomer base. Table 6 and Figure 1 show the performance spectrum of such coatings for four representative OH-polyacrylate grades in combination with a standard polyisocyanate. Due to the lower OH content and its low-cost monomer composition, Grade 1 offers interesting system costs, but compromises on the weathering and chemical resistance of the coatings. Whereas Grade 2 contains lower-cost materials and achieves weathering results comparable to the conventional standard, the system costs are relatively high because of the high OH content. With a comparatively low OH content, Grade 3 offers balanced performance – which is superior to the standard in terms of weathering – but it contains expensive monomers.
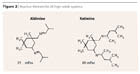
To accelerate the generally slower drying time of high-solids systems, the formulations are often catalyzed. However, this step further reduces the already short pot life. Reactive thinners are a possible alternative (Figure 2). These are usually aldimines, or in rare cases ketimines, which only slightly shorten the pot life but are a disadvantage in practice because of their odor.9As modern, odorless alternatives, polyaspartics can be used as reactive thinners in polyurethane paints. Recently, this technology has also become increasingly widespread as the sole binder for modern anti-corrosion coatings and is therefore discussed separately under “High-Solids Polyurea.”
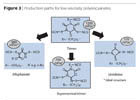
Modern polyisocyanates likewise contribute to high-solids systems (Figure 3): assuming a classical polyisocyanate trimer, the percentage of uretdiones and allophanates can be controlled in production by using catalysts to specifically reduce viscosity. Whereas in the past a loss of functionality was accepted, new catalysts now make it possible to generate a percentage of the asymmetrical trimer that significantly reduces viscosity while maintaining functionality.
High-Solids Polyurea
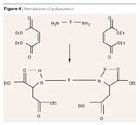
Conventional non-solvent polyurea topcoat binder systems based on amine plus aliphatic polyisocyanate are highly reactive and can only be applied hot with special 2K spray apparatus in film thicknesses upwards of 500 μm, which greatly restricts their use for corrosion protection. Adding a solvent is not an alternative, as it cannot escape from the film because of the high reactivity (blistering).Bayer’s polyaspartics represent a relatively new product class. They are sterically hindered, blocking agent-free, secondary aliphatic amines with specifically reduced reactivity to polyisocyanates. They require no corresponding labeling (non-corrosive), are virtually colorless, and have only a mild odor. In production, they are obtained by the Michael addition of sterically hindered amines with maleic acid diester (Figure 4).
Several polyaspartic grades are available in Europe for the manufacture of pigmented, high-solids anti-corrosion topcoats that have pot lives ranging from one minute to four hours and thus permit a variety of application methods (spraying, rolling, brushing) (Table 7).
The advantages of these systems in anti-corrosion applications have already been published in detail.10To summarize, they are characterized by high processing reliability (high film thicknesses can be applied without blistering), high-efficiency (rapid drying, outstanding anti-corrosion properties even in two-coat systems, good reworkability and repairability) and very low solvent contents (sprayable up to 90 g/l VOC). One limitation is the relatively expensive raw material base compared to the high-solids polyurethane technology described above.

Application efficiency is very crucial when coating steel parts with large surface areas, such as the cement/gravel processing plants shown in Figure 5: a two-coat system was selected comprising 1x60 μm 1K polyurethane zinc dust primer and 1x60 μm 2K polyaspartic topcoat. The 1K primer offers the advantage of a short intermediate drying time (eight hours at 10 ºC, compared to 24 hours for the standard EP-zinc dust primer), meaning that the topcoat could be applied the same day, allowed to dry overnight, and the parts could already be moved the next day. Only 37 g solvent per m2coating were emitted for the total system; the standard system (conventional EP/2K polyurethane) would have emitted 93 g/m2. Moreover, the topcoat displays improved low-temperature flexibility, which benefits operation in winter.
The technology is associated with two limitations: while it offers an advantage in terms of application efficiency thanks to the reliable application of high film thicknesses, an accelerator (usually aldimine) must be added to topcoat formulations with a fairly long pot life (i.e. those suitable for rolling and brushing) to ensure thorough drying. Aldimine, however, leads to a certain degree of gloss reduction when exposed to the effects of weathering, as shown in Figure 6.
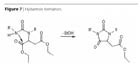
Another limitation is thermal stability. If the binder systems are exposed to temperatures above their glass transition temperature for an extended period of time (Table 7), hydantoin formation, i.e., ring closure associated with the separation of ethanol, may occur (Figure 7), resulting in a certain degree of shrinkage. This applies specifically to clear coats (thus excluding use in the automotive sector). In contrast, pigmented/filled systems are only affected at higher temperatures (i.e. 10 to 20 ºC above Tg). In roughly eight years of practical use in anti-corrosion applications, no negative effects of hydantoin formation have been reported. In terms of its application versatility and price/performance ratio, this class of binders offers interesting prospects.

High-Solids Polysiloxane
Functionalization with silanes is a development issue affecting virtually all binder classes in the 1K and 2K sector. To date, practical experience has been acquired with 2K EP-polysiloxane hybrids and 1K acrylsiloxane (primarily restricted to use as a refinishing system). Only the 2K epoxy siloxane hybrid system is discussed here, as it is the foremost competitive system in this application.The advantages include high efficiency (high film thicknesses with rapid drying rates at normal temperatures, thus permitting two-coat systems similar to the polyaspartics), low VOC content (typically 120 g/l solvent for a sprayable system) and excellent chemical and weathering resistance. The very high comparative binder costs and limitations on repairability are prohibitive for acceptance in the classical corrosion protection market. However, there has been some experience in niche applications involving extreme conditions, e.g., the offshore sector. Under such unusual conditions, several limits of the system become apparent, although they can hardly be compared directly with those of other modern systems because the suitability of modern systems for use under such extreme conditions has not been sufficiently investigated.
Most are hard coatings, which if applied in high film thicknesses, tend to crack when subjected to mechanical load due to their limited flexibility. According to the datasheet, the pot life is approximately two hours under standard conditions, although that is difficult to control in view of the changing conditions at a construction site. Moreover, intercoat adhesion to zones that have already cured can cause problems – a situation that imposes high demands on applicators. In individual cases, pigmentation with critical color pigments can cause color stability problems during weathering with this fledgling class of binders. Figure 8 shows a comparison of a polyaspartic and polysiloxane during artificial weathering over an extended period. Hardly any differences can be determined between the two systems.

Waterborne Coatings
Water-reducible topcoat systems – essentially based on 2K polyurethane (polyurethane dispersion), non-functional polyacrylates (polyacrylate dispersion), or mixtures thereof (polyurethane/polyacrylate dispersion) – have not succeeded in becoming significantly established in the field of corrosion protection. The market share of water-reducible systems that cure at room temperature is below 5% in Europe. Similarly, they have only been declared "limited state of the art" in the revised version of ISO 12944. The main fields of application are: general industrial coating, heavy-duty vehicle coating and the ACE sector. It must be kept in mind for all waterborne coatings that the results of accelerated laboratory tests frequently do not correlate with practical experience. Raw material manufacturers in particular are currently working at full steam to develop more durable binder systems.Coatings based on modern polyurethane dispersions are characterized by a low VOC content (usually about 130 g/l VOC from co-solvents at application viscosity). Under optimal curing conditions (forced drying following controlled evaporation), they achieve the property profile required for up to corrosiveness category C3 as per ISO 12944. In general, forced drying can be ruled out for anti-corrosion applications in the construction sector discussed here. Consequently, achievable coating hardness levels are still limited (maximum pendulum hardness: approx. 70 s).
The non-crosslinked polyacrylate dispersions are less expensive than polyurethane topcoats, but display a lower performance level in terms of their anti corrosion properties. This also applies to application robustness.

Powder Coatings
Powder coatings based on various binders are used in anti-corrosion applications, from sintering powders based on a special polyolefin in light-duty corrosion protection (and in heavy-duty corrosion protection in three-coat pipeline coatings), to polyurethane and polyester powders up to EP/polyester hybrids and fusion-bonded epoxy (FBE) powders in heavy-duty corrosion protection (primarily pipeline construction and rebar applications). All of the coatings are baking systems with the advantage of containing no solvents. However, the trade off is the significantly higher amount of energy required for their application compared to solventborne or water reducible systems that cure at room temperature. In addition, investment costs for the required equipment are high. Powder coating technology is widely used in stationary installations for coating high volumes of primarily small components (exception: the pipeline sector with the FBE coating of pipes up to 2 m in diameter and 12 m long!). A powder coating based on an EP/polyester hybrid, which is used for corrosion protection in construction applications (steel profiles, hollow bodies), cures at roughly 180 ºC. The coated, solid steel parts cannot be handled manually until after about four hours. To compare, quick-drying 2K polyaspartic (Grade 2) topcoats can withstand foot traffic after just 10 minutes. Repairability is also limited when highly crosslinked powder coatings are used.Powder coatings are also encountered in heavy-duty corrosion protection as duplex systems on hot-galvanized steel. A new corrosion protection standard is being drafted to segment the recently increasing complexity of powder coating systems with respect to their suitability for anti-corrosion applications.

Conclusions
- High-solids polyurethane coatings meet VOC requirements and nearly match the conventional polyurethane coating standard.
- Polyaspartic coatings fulfill both heightened VOC and efficiency requirements, while simultaneously offering price/performance advantages.
- Polysiloxane coatings fulfill the more stringent VOC requirements and display high-level performance, but applications are still restricted to high-quality niche areas.
- Waterborne and powder coatings fulfill the higher VOC requirements and, because of their properties, are largely limited for corrosion protection purposes to stationary application installations.


Report Abusive Comment