
Coatings have been UV cured in industrial settings since the 1960s. The graphics industry was one of the first to adopt this technology, with a high-gloss coating on cards. Today, there are numerous industrial applications that utilize UV curing as the method of drying or polymerizing their coatings or inks. It is only in the past five years or so that commercial UV curing has moved out of the factory and into the field. Floor coatings are one of the main applications for field-applied UV-cured coatings. Today, floor coatings for concrete, wood, vinyl and tile are all in some phase of commercialization. This paper will introduce the basics of UV curing, and provide structure property relationships for UV-curable materials. Several starting-point formulations for UV-curable concrete coatings will also be provided.
UV Curing Basics
AdvantagesUV curing is also known as radiation curing or energy curing. It utilizes the ultraviolet (UV) portion of the electromagnetic spectrum, 200 - 400 nanometers (Figure 1), to physically dry or cure coatings. (The coating actually polymerizes or increases in molecular weight during the curing process.) To do this on an industrial scale, lamps that emit UV light replace drying ovens, and the drying takes place in fractions of a second. Fast cure speeds are not the only benefit of UV curing. The physical space and energy requirements for the drying are also greatly reduced versus conventional drying mechanisms. Because UV-curable coatings are 100% solids, VOCs and HAPs are also very low, generally less than 5% VOC content. Since solvents are not typically used in UV-curable systems, inhalation and flammability hazards are essentially non-existent.

The UV light (hn) must be absorbed by the coating in order for curing or polymerization to occur. This step is accomplished by photoinitiators (PI), which react to form free radicals (PI˙). These free radicals then react with the double bonds in the coating (typically acrylate double bonds, Figure 2) to form new free radicals (PIR˙) in an initiation step. The propagation step causes the coating molecular weight to increase through chain reactions with additional acrylate double bonds (R2). As the molecular weight increases, the coating becomes less fluid or mobile, and begins to solidify. The chain transfer step controls the molecular weight increase by terminating one chain reaction, and transferring the free radical to begin a new chain reaction. The termination step ends all reactions and stops molecular weight increase. Figure 3 shows these various steps in the free radical chain polymerization process.

During the free radical polymerization, the coating becomes crosslinked because the acrylate molecules contain multiple double bonds. The number of acrylate double bonds in a molecule is called its acrylate functionality. For example, if a molecule contains two acrylate double bonds, it is difunctional. The degree of crosslinking of a coating indicates how tightly the polymer network is interconnected. This crosslink density impacts the coating properties. A more highly crosslinked coating will be hard and chemical resistant; a less crosslinked coating will be flexible and soft. The weight per double bond of a coating formulation predicts the crosslink density, and thus the coating properties. Table 1 illustrates this concept. The weight per double bond is calculated by dividing the average molecular weight of the formulation by the average functionality of the coating. (It should be noted that this is only a predictive tool, and that factors other than crosslink density also affect coating properties.)
Cure Speed
Cure speed is the line speed at which the desired coating properties are obtained. The desired coating properties can and do vary based on the end use of the coating. For an overprint varnish, this may be 10-20 MEK double rubs. For a wood floor coating, this may be 200+ MEK double rubs and Shore D hardness of 70. For coatings for food packaging, this may be a migration level of less than 50 ppb. In most cases, the acrylate double bonds in a coating are not completely consumed. Consumptions of 80-90% are typical since the vitrification of the coating limits the mobility of the free radicals in the latter stages of the reaction. Since there are multiple acrylate double bonds on most components in the formulation, these consumption rates do not necessarily indicate raw materials that are not part of the polymer network.
As discussed in the previous section, cure speed is impacted by the weight per double bond of the formulation. The lower the weight per double bond (or the more acrylate double bonds per molecule), the higher the cure speed. Cure speed is also affected by a phenomenon known as oxygen inhibition. Molecular oxygen is a very reactive material that can react with the photoinitiator and polymer radicals to form peroxy radicals, which then slow down the free radical chain reaction. Severe oxygen inhibition manifests itself as a thin layer of unpolymerized (liquid) material on top of the coating. Less severe results are reduced coating properties, such as decreased hardness and chemical resistance.
Oxygen inhibition can be mitigated in several ways. The use of inert atmospheres, such as nitrogen, removes oxygen from the curing process. Higher UV lamp intensities and higher levels of photoinitiator increase the concentration of photoinitiator and polymer radicals versus that of the oxygen-based peroxy radicals to reduce the amount of oxygen inhibition. Photoinitiators that absorb at shorter wavelengths are particularly useful. Finally, amine synergists can be added to the formulation to consume oxygen, and thus reduce its effects.
Oxygen inhibition is mainly a surface cure phenomenon, since dissolved oxygen is quickly consumed and not easily replaced. Through, or deep, cure of a coating is affected by other issues, and is generally manifested by lack of or decreased adhesion and decreased solvent resistance. To mitigate through cure issues, photoinitiators that absorb at longer wavelengths are used. The photoinitiator concentration should generally be lower for good through cure. (The UV light must not be completely absorbed at the surface of the coating, which may occur at high levels of photoinitiator.)




Combinations of photoinitiators, which absorb at different wavelengths, are often used to overcome many of these pigment absorbance issues. For each formulation, different types and combinations of photoinitiators should be evaluated, and ladder studies for photoinitiator concentration should be run to optimize the cure of the coating.
UV-Curable Raw Materials
Acrylate OligomersThere are two main classes of acrylate raw materials: oligomers and monomers. Oligomers are also known as prepolymers, and are generally high in molecular weight (500-5000). They provide much of the overall coating performance. Coating properties such as hardness, abrasion and scratch resistance, chemical and solvent resistance, flexibility, and toughness are greatly impacted by oligomer choice. The number of acrylate double bonds per oligomer molecule (acrylate functionality) varies from 2-6. Oligomers are divided into three main classes by their chemical backbones: epoxy acrylates, urethane acrylates and polyester acrylates.
Epoxy acrylates provide coatings that are hard, chemical and temperature resistant, glossy, and fast curing. Coatings based on epoxy acrylates do not weather well because of their aromatic character. This oligomer is generally the highest viscosity, the lowest cost, and is almost always difunctional. Figure 8 shows the most commonly used epoxy acrylate.
Urethane acrylates provide coating toughness and abrasion resistance. They can vary in hardness and flexibility, depending on their composition and acrylate functionality (2-6). Urethane acrylates are moderate to high in viscosity and provide coatings with moderate cure speed. Aliphatic urethane acrylates provide coatings with the best weatherability.

Table 2 summarizes the properties of the different oligomer classes on a relative basis. This is a general summary, and there can be substantial deviations, depending on the specific oligomer.
Acrylate Monomers
Since oligomers are high in molecular weight, they are quite viscous and generally require monomers as diluents to obtain the desired application viscosity. Monomers are also known as reactive diluents since they contain double bonds and react to become part of the polymer during free radical polymerization. Since monomers become part of the coating, the flexibility or hardness of the coating can be impacted by monomer choice. They are generally low in molecular weight and viscosity. Their acrylate functionality varies from 1-6, and they are generally categorized by their functionality.
Monofunctional monomers contain one acrylate double bond per molecule. They are the lowest-viscosity monomers and the best viscosity reducers for oligomers. Monofunctional monomers reduce crosslink density, and result in flexible coatings with better substrate adhesion. Since they only have one acrylate double bond, they reduce the cure speed of formulations, and may result in unreacted monomer that is potentially extractable.

Tri- and higher-functionality monomers are typically higher in viscosity, and are not as effective in reducing the viscosity of the oligomers. They increase the cure speed of a coating, but can also impart brittleness to the coating. Increased crosslink density typically improves hardness, abrasion and scratch resistance, and chemical and solvent resistance.

Health and Safety
Acrylate functional raw materials are non-toxic and have very low volatility at room temperature. Therefore, the inhalation hazard is very low, and respiratory protection is generally not necessary. Acrylates may be skin and eye irritants, so protection such as safety glasses and gloves are required. Additionally, protection from exposure to UV light may be required, and is provided by tinted safety glasses and sunscreen for exposed skin areas.

UV-Curable Coatings
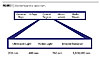
Coating BasicsA UV-curable coating must contain several basic materials in order to undergo effective curing or polymerization. Additional materials are also required to obtain the desired coating aesthetics. The coating pyramid is often used to demonstrate the use of these materials (Figure 10). Materials at the base of the pyramid generally comprise more of the formulation than materials at the top of the pyramid. The base of the pyramid contains the oligomers, which, as discussed earlier, provide the bulk of the coating properties. Next on the pyramid are the monomers, which are primarily used to obtain the desired application viscosity. Additives are used to enhance the coating performance and/or to change the coating appearance. As in conventional coatings, these additives can be flatting agents, fillers, wetting/leveling agents, defoamers or slip aids. Pigments can also be added to a formulation for color or special effect. Photoinitiators are needed to absorb UV light and form free radicals.

A UV-curable concrete coating must be low in viscosity, around 150 cP, to allow air release upon application of 1-7 mils coating thickness. This low viscosity requirement limits the choice and/or concentration of oligomers in a formulation. Monomer choice also becomes more important since the formulation will contain a large amount of diluting monomer. A combination of photoinitiators should be used to ensure both surface and through cure. Flow, leveling and wetting agents, and defoamers may be necessary for coating aesthetics. See Table 4 for a generic starting point formulation for a UV-curable coating for concrete.
The effect of oligomer choice on concrete coating properties was evaluated using the actual starting point formulation given in Table 4. Epoxy acrylate (EA), urethane acrylate (UA), and polyester acrylates (PEA 1 and PEA 2) were evaluated as the oligomer. These oligomers were chosen because of their low viscosity and beneficial properties for concrete coatings. Various coating properties, such as cure speed, gloss, adhesion, hardness, flexibility, chemical resistance and abrasion resistance were measured. These coating properties were then correlated to structural elements of the oligomer.

Experimental Details
MaterialsSmooth concrete blocks (4 x 6 x 1 inch) were obtained from Patio Concrete Products, Inc., and were wet wiped and then dry wiped to clean the surface before coating. Oligomers, monomers and photoinitiators were obtained from Cytec Industries Inc. and used as received. Additives were obtained from Ciba and Air Products, and were used as received.
The coatings were cured using a laboratory cure unit from HID Ultraviolet, LLC., which consists of a mobile curing unit (Bulldog 15-3000) equipped with a 250 watts per inch medium pressure continuous wave xenon lamp. It is mounted on a conveyor system with variable belt speed (Dynomometer) to enable ease of laboratory work. The curing unit was calibrated using a 390 Belt Radiometer from International Light Technologies.
Procedures
An Instron model 4467 was used to determine tensile properties on 6-7 mils thick, UV-cured films of the oligomer containing 4% 2-hydroxy-2-methyl-1-phenyl-propanone as the photoinitiator.
The coatings were prepared by mixing under agitation all components shown in Table 4, except the oligomer for coating properties and the defoamer. The oligomer, which had been heated overnight at 65° C to reduce viscosity, was then added under agitation, followed by the defoamer. The viscosity of the resulting formulation was measured at 25° C using a Brookfield DV-II + viscometer equipped with a #21 spindle. Viscosity of the UA and PEA 2 formulations was adjusted by adding 7% of the viscosity reduction monomers, and the EA formulation was adjusted by adding 5%. Final viscosities were then measured in the same manner as noted above.
Coatings were applied to the concrete blocks with a #25 Meyer wire wound rod, to obtain a coating thickness of 5-7 mils, as measured with a Gardco wet film gauge.
The coatings were then cured at various exposures (mJ/cm2), corresponding to various walking speeds (feet per minute, fpm). The minimum exposure required to obtain a mar-free surface as determined with a wooden tongue blade was recorded as the cure speed. Coating properties at the cure speed were then determined as shown in Table 5. Additionally, all coatings were cured at 919 mJ/cm2 (20 fpm), in order to compare coating properties at the same cure speed.

Results
The oligomer tensile properties are given in Table 6, and the stress-strain properties for the oligomers are graphed in Figure 11. Coating properties are shown in Table 7.
Discussion
The stress-strain properties shown in Table 6 and Figure 11 indicate that the epoxy acrylate and urethane acrylate are tougher than the two polyester acrylates. The epoxy acrylate has been modified to be more flexible than the standard bisphenol A-based epoxy acrylate, and this is reflected in its tensile properties that differ from the typical properties of epoxy acrylates. The urethane acrylate shows toughness expected for its oligomer class. The two polyester acrylates show expected results as well.


The relative weatherability and oligomer cost is shown in Table 9. If the concrete coating will be exposed to sunlight and requires excellent weatherability, the urethane acrylate is the oligomer of choice, although at a higher cost. The two polyester acrylates can be used in areas where the need for weatherability is not extreme, and at a better cost than the urethane acrylate. The epoxy acrylate should only be used in areas where weatherability is not a concern.
Conclusion
Three classes of oligomers have been evaluated in coatings for concrete. The low viscosity requirement of the concrete coatings minimizes the effect of oligomer choice on final properties, and all oligomer classes provide coatings with high gloss and good chemical and solvent resistance. However, the PEAs do provide better hardness at reasonable cure speeds, along with a good compromise on weatherability and cost. PEA 1 provides the best combination of properties: cure speed, adhesion, hardness, weatherability and cost.Acknowledgements
The author would like to thank Jim Smith, Kurt Willard and Angela Carmack, all of Cytec Industries Inc., for their assistance in the preparation and evaluation of the coatings.
The paper was presented at the FSCT 2009 Advancements in Coatings Series: “Coating” the World of Concrete held February 2, 2009 in Las Vegas, NV.




Report Abusive Comment