Thermal-Cure Urethane Prepolymers

|
| Figure 1 Click to enlarge |
Material science and chemistry of polyurethanes (PUs) have matured due to extensive studies that began in 1937. Many books and review articles are devoted to this subject.(1-3) There is a task involved in the formation of PU with required properties in a certain mold, or production of PU of a certain shape. Simple examples are manufacturing of PU shoe soles, PU belts or an application of a PU coating. Evidently one needs to have a liquid (flowable, pourable) urethane prepolymer, which will be cured in a form or on a support, forming a PU product.
Typically urethane prepolymers are based on the reaction of a polyol with an excess of di- or other polyisocyanate. The forming prepolymer has a certain calculated and/or experimentally measurable concentration of non-reacted isocyanate -NCO groups.
The typical concentration of –NCO groups (FW= 42 g/mol) is 5-10%. The proper amount of curative, which is usually an organic difunctional compound with hydroxyl or amino groups, is added to the prepolymer (Figure 1).
The problem, well-known to chemists and manufacturers, is to mix homogeneously and quickly the prepolymer with the curative. Some common curatives are liquids like 1,4-butanediol or 2-aminoethanol. However, many curatives are solid compounds at room temperature with tm > 100 ºC. Also, the prepolymer, a high-MW compound, is viscous at room temperature. In order to reduce the viscosity of the prepolymer for excellent mixing with a curative, the prepolymer should be hot. Then, there is the danger that during mixing at elevated temperatures the prepolymer and curative will react prior to filling the form with a reactive mixture. A mold filled with a reactive mixture is left for many hours at high temperature for cure. There is a known way to use blocked isocyanate prepolymers, which are unstable adducts of –NCO with a blocking agent that dissociate at a high cure temperature. Blocked prepolymers allow a thorough mixing of non-reactive blocked prepolymer with a curative at a high temperature but lower than the characteristic temperature of deblocking.(1-3)
The PU industry uses so-called low free (LF) monomer prepolymers. These are prepolymers with no unreacted free polyisocyanate. That way the prepolymer is less hazardous than a common prepolymer, which has a low concentration of non-reacted hazardous volatile diisocyanate. In particular COIM USA produces different urethane prepolymers including LF prepolymers.(4) This brief introduction does not aim to address the recent advances in this area.
Exposure of a thin layer of urethane prepolymer to the moisture in the air leads to the slow formation of polyurea coating.
Radiation Cure of Prepolymers
It was described above that thermal cure of urethane prepolymers is a time- and energy-consuming process. We have also mentioned the difficulty of quickly mixing reagents at elevated temperatures. There is an alternative way of curing urethane prepolymers – radiation cure. (The word oligomer is often used in the radiation curable industry instead of the word prepolymer.) Urethane oligomers are irreversibly capped with polymerizable molecules, such as hydroxy-substituted acrylates, hydroxy-substituted methacrylates, hydroxy allyl ethers or hydroxy vinyl ethers in order to make urethane oligomers that are radiation curable. Oligomers with double bonds can undergo radiation-induced free-radical polymerization. The most common capping agents are 2-hydroxyethyl acrylate (HEA) and 2-hydroxy methacrylate (HEMA).
Under UV cure, UV light excites special additives called photoinitiators (PIs), which undergo photochemical reaction leading to the formation of free radicals. Usually cure takes place at room temperature. Cure (photopolymerization) is an exothermic reaction, and it is accompanied by the release of heat. Radiation-curable urethane acrylate coatings find multiple applications in graphic arts, electronics, furniture, and manufacturing of optical fiber, optics and adhesives.

|
| Figure 2 Click to enlarge |
UV-Curable Urethane Acrylates
Acrylate oligomers are prepared the usual way by a reaction of polyol with polyisocyanate. Commonly used polyols are polyethers, polyesters and polycarbonates. In recent years, silicone carbinols, polybutadiene and hydrogenated polybutadiene (polyethylene) diols (bio-based polyols) are used. The common OH functionality of polyols is 2 or 3. The polyisocyanate industry widely uses diisocyanates with known abbreviations such as IPDI, TDI, H12MDI. Common triisocyanates are HDT and Desmodur N100.
Usually oligomers are prepared the following way: diisocyanate reacts with polyol and such oligomer is capped by, for example, HEA. This is called direct addition.(5) One can react diisocyanate with HEA first, and after that react a product with polyol. This method is often called reverse addition. The properties of formed urethane acrylates obtained by direct and by reverse addition are quite different even under the same stoichiometric ratio of the three reagents. This issue is discussed in more detail elsewhere.(5)
A standard coating formulation consists of urethane acrylate oligomers that determine the properties of the cured film and reactive diluent, which is a mono or diacrylate for reducing the viscosity of the oligomers and ease of handling of the formulation. There are expectations concerning what properties of the cured coatings the diluent contributes. Popular diluents, like 2-phenoxyethyl acrylate (PEA) or isobornyl acrylate (IBOA), usually increase elasticity of the cured films, whereas 1,6-hexanediol diacrylate (HDDA) imparts hardness to the cured films. In a number of cases diluent addition is demanded only by reduction of viscosity. UV-curable urethane acrylates are solventless and lead to practically zero VOCs.
PIs are usually the only low-MW compounds that do not become part of a forming polymer chain in the polymer network of a cured coating. Therefore, PIs sometimes create difficulties: they can migrate and exit a thin film, and products of their photo-induced reactions have odor. A method suggested below alleviates these drawbacks. Figure 2 shows a UV processor used for fast cure of a radiation-curable oligomer/formulation.
|
| Figure 3 Click to enlarge |
LS Oligomers
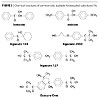
It was stated above that the main part of a UV-curable urethane acrylate coating is the urethane acrylate oligomer, which is formed by the reaction of polyol with isocyanate, i.e., by a reaction of –OH with –NCO groups. A number of available PIs have primary, secondary or tertiary aliphatic hydroxyl groups (Figure 3), and they can react with the isocyanate functionality of the oligomer.
We used the latter route for synthesis, and we grafted the PI onto the urethane acrylate oligomers. Such oligomers, with grafted PI, were named UV-Light Sensitive (LS®) oligomers.(6-9)
Benzoin was used as the PI. In our experience, benzoin is a very efficient photoinitiator despite a relatively long-lived excited triplet state compared to some other PIs of the same family. The photochemistry of benzoin and reactivity of corresponding free radicals are well documented.(10)

|
| Figure 4 Click to enlarge |
Figure 4 is a pictorial presentation of polymerization initiation of a difunctional acrylate oligomer by a grafted and dissolved Type I PI.
Evidently, LS oligomers with 0.5 - 5.0 wt.% of grafted PI have slightly less concentration of acrylate groups than the conventional analogue. Grafting at a level of less than 5 wt.% produced minor effects on the properties of liquid and cured coatings.
Diols like Irgacure 2959 and Irgacure 127 (Figure 3) can be used as chain extenders, and they can be grafted by two OH groups. The primary hydroxyl group in Irgacure 2959 is more reactive than the tertiary hydroxyl.
Oligomers endcapped by Irgacure 2959 at the primary OH group were prepared. Photolysis of such oligomeric PI results in formation of a substituted benzoyl free radical bound to a chain and free 2-hydroxy-2-propyl radicals. An increase of oligomer chain results in a slower tumbling of the benzoyl fragment. The latter suggestion explains the increase in intensity of an ESR signal of substituted benzoyl radical and an increase of relaxation time T1 of the benzoyl radical measured in FT-TR ESR experiments with oligomeric PI.(11)

|
| Table 1 Click to enlarge |
Our experiments demonstrate that a migration and leachability of the residual PI profoundly decreases from cured coatings with LS® oligomers.9 That was expected for grafted compounds.
Examples of Urethane Acrylate Oligomers
Chemists familiar with formulating urethane prepolymers have the bulk of the knowledge required to formulate urethane acrylate oligomers, allowing for a short learning curve. As with their conventional counterparts, urethane acrylate oligomers derive their properties of tensile strength, elongation, hardness, weatherability, adhesion, etc. from the interplay of polyol and isocyanate selection, with the added minor complexity of the specific nature of the chosen acrylate end-cap group.
As with thermal-cured urethanes, urethane acrylate oligomer properties can range from the hard segment materials commonly used in wood coatings to the soft, tacky, high elongation-to-break used in film laminating and pressure-sensitive adhesives. We present below names of oligomers manufactured by Bomar Specialties.(6)
In the case of BR-144, a low/medium-molecular-weight polyether urethane tri-acrylate oligomer provides the same properties that a similar non-acrylated prepolymer would – namely high hardness, scratch and abrasion resistance, outdoor durability, and extensive chemical resistance.
BR-7432G is a difunctional of a higher molecular weight, designed for both high elongation and tensile strength. This material has lower unsaturation and reduced shrinkage upon cure, a problem with highly unsaturated urethane acrylate oligomers.
BR-3641AA, by comparison, is a low-functionality (1.3, average), high-molecular-weight material with high hydrocarbon content and, as such, provides an opposing extreme of elongation and tackiness.(12) Table 1 compares a selection of physical properties of these three oligomers, diluted in a common acrylate monomer.
A large number of urethane acrylate oligomers are available that offer combinations of properties that vary between and beyond these two extremes.6 In addition, the choice of acrylate end-cap allows for some unusual modifications to the urethane prepolymer. An excess of acrylate functionality, as with the ~15 acrylate groups of BR-101D, results in an additive urethane acrylate oligomer that can further improve the specific properties of scratch resistance and hardness, by introducing areas of extremely high crosslink density.
Likewise, the backbone itself can be selected from unconventional chemistries, as with the silicone backbone of BRS-14320. Here, a silicone backbone, incorporated into a flexible urethane diacrylate prepolymer, improves the property of heat resistance and good compression-set ratio in a low-crosslink-density oligomer, offering the possibility of a final formulation that incorporates properties more typically associated with silicone rubber. This product also exhibits the blend of low shrinkage and overall toughness of a possible candidate for cast applications.

|
| Figure 5 Click to enlarge |
UV vs. EB
Electron beam (EB) cure is an irradiation of a polymerizable formulation by high-energy electrons. Such irradiation creates free radicals, which initiate polymerization of acrylates and other “ene” compounds. There is an ongoing discussion between proponents of UV- and EB-cure technologies. EB equipment is more expensive that UV processors or other UV or visible light sources. At the same time EB cure does not require PIs and lacks the issue of light penetration into colored coatings or coatings with a strong absorption in the UV part of the light spectrum (UV cure.) In the case of UV cure, one needs to deal with PIs. The use of PIs may have some drawbacks as described above, therefore self-cured formulations that do not require an addition of PI are of interest. Such formulations include in particular chemically bound maleimide derivatives, vinyl acrylate, thiols and benzophenone fragments in the chain.
Magnetic and Spin Effects Under UV Cure
Free radical photopolymerization is accompanied by formation of transients with a non-zero spin angular momentum that play a role in polymerization: i.e., free radicals, radical pairs, triplet states of photoinitiators, and a stable molecule in a triplet state (molecular dioxygen). One can expect a manifestation of magnetic and spin effect in free-radical photopolymerization.
Magnetic and spin effect was a hot topic in physical chemistry and photochemistry two to three decades ago. A number of mechanisms of magnetic field (MF) effects in chemical reactions were established, well-documented and got a proper theoretical analysis. Still an application of MF effects to photopolymerization is rather limited and deserves further exploration. It was proven in a number of experiments that applications of a moderate MF accelerate free radical polymerization.(13)
UV Cure of Sizable Articles
Fast UV cure of urethane acrylate coatings is a well-developed technology. Cure of sizable objects, especially for rapid prototyping, is a rapidly developing area.(14) Objects that are thermal cured in a mold can be cured by UV light. It was demonstrated that with bleaching photoinitiators like phosphine oxides one can UV cure large objects by irradiating them usually from the top. There is a report on the cure of a 25-in (length) post irradiated by a UV light thiol (PI) containing material from the top.(15) Hoyle et al.(16) developed UV-induced frontal polymerization of multifunctional (meth)acrylates. Polymerized material was placed in a test tube with a length of 4.9 in. UV light-induced frontal polymerization can be performed on colored, even black materials.
Conclusions
We hope that some manufacturers of PU products will find radiation cure of urethane prepolymers (oligomers) as an attractive alternative of thermal cure. They may find that an approach such as the one illustrated in Figure 5 is more attractive than the traditional approach shown in Figure 1.
Radiation cure, and in particular UV cure, has many advantages vs. thermal cure, such as speed, low energy consumption, and temporal and spatial resolution. An association of chemists and manufacturers working in EB and UV cure, RadTech,(17) had, for a number of years called their conferences “e/5”, which stood for efficient, enabling, economical, energy saving and environmentally friendly.
Summary
This brief review article did not aim to cover the most recent advances in the area of radiation cure and thermal cure of urethane prepolymers (oligomers) but rather briefly describe the current status of this technology.
UV processors are affordable, with a starting price of $5 k. A simple, inexpensive processor fits inside the trunk of a car. Although not necessarily UV, cure with visible light can be rather efficient. An excellent example is a cure by visible light (l 450-480 nm) of dental restorations, which are methacrylates and urethane methacrylates in particular. Formulations cured by visible light are colored; that color may decrease or disappear due to the partial or complete consumption of PI with the formation of colorless products.




Report Abusive Comment