However, alternative surfactants are desired to expand the formulators’ surfactant options and give materials that are free of APEOs. Previous works showed that narrow-range alcohol ethoxylates, based on different hydrophobic feedstocks, are effective APEO alternatives as nonionics in emulsion polymerization. By exploiting the nonionic work, new APEO-free ether sulfates have been developed that yield emulsion polymerization characteristics similar to that of the APEO-based ether sulfates. Thus, the following work compares and contrasts standard industrial APEO anionic emulsifiers with new non-APEO-based alternative anionic surfactants in model systems.
|
| Figure 1 Click to enlarge |
Introduction
Alkylphenol ethoxylates and ether sulfates in general possess a number of structural, compositional and performance attributes that have helped them to reach high consumption rates for emulsion polymerization in North America. A number of these advantages are listed in Table 1.

|
| Table 1 Click to enlarge |
In 2006, the total surfactant usage for emulsion polymerization was 106,400 metric tons (calculated as 100% active), which is a modest 3% growth over volumes used in 2004. This has slowed dramatically over the recent economic downtown; however, the outlook for growth for emulsion polymerization is still positive over the next five years. Of the 2006 volume, nonionic surfactants made up 55,000 metric tons or >50% of the total surfactant volume, while anionics took up most of the remainder with 51,400 metric tons.(3)
Sales of APEO-based surfactants accounted for most of the total nonionic surfactant volume, including 22,700 metric tons octylphenol (OPEs) and 17,700 metric tons nonylphenol (NPEs) ethoxylates as nonionics. Additionally, the APEO surfactants accounted for another 8,200 metric tons as anionics, of which much was nonylphenol ether sulfate (all numbers calculated at 100%).(3) These particular nonionics and anionics were preferred for emulsion polymerization because they provided cost effectiveness and improved particle stability over a wide range of thermal, mechanical and electrolyte conditions via steric stabilization for the nonionics and charge stabilization for the anionics.(1,4)
Recent Trends for Substitution of APEOs
Despite all of the aforementioned advantages for APEOs and the sulfate analogs, their use in North America has started to decline. The trend, which has been dramatic over the past two years for household detergent applications, is now moving to many industrial applications in North America. Some of this is due to governmental pressure.

|
| Table 2 Click to enlarge |
Like the EU (European Union), which has already banned the use of APEOs for applications where these surfactants could contact sewer water, the Canadian government is starting to regulate APEO use. In fact, Canadian legislation is requiring a 90% replacement of all APEOs including their derivatives like sulfates within products consumed in Canada by 2010.(5,6) In contrast, the pressure in the United States is not coming so much from the federal government as from large merchandisers such as Wal-Mart and Home Depot. They are encouraging their suppliers of all consumer products (household detergents, paint, etc.) to use eco-friendly components within their product formulations. As a result, use of NPEs and NPE sulfates, in particular, are being phased out.(7,8) Table 2 shows some of the disadvantages of APEOs, which are driving their reduced use in industrial applications.
In addition to the disadvantages, the cost/performance advantage that APEOs and the derivatives have historically enjoyed has come under pressure due to higher petroleum prices and limited availability of the feedstock propylene trimer, which is used to manufacture the nonylphenol hydrophobe.(9,10)
Previous APEO Alternatives

|
| Figure 2 Click to enlarge |
In prior work on emulsion polymerization, nonionic surfactant alternatives to APEOs were examined. The results of this research suggested newer hydrophobes, including isotridecyl alcohol (TDA) based on n-butene feedstock and Fischer-Tropsch (“FT”) process-based alcohols (FT-OXO) with carbon range of 12 to 13 (Figure 2), were a better match for the properties of octylphenol and nonylphenol than other alternatives in the marketplace when coupled with narrow-range ethoxylation. Nonionic surfactants derived from these hydrophobes and narrow-range ethoxylation resulted in equal or better performance than NPE or the standard OPE in a standard emulsion polymerization.
Applying Previous Knowledge

|
| Figure 3 Click to enlarge |
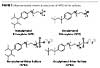
The previous work, again, centered on the nonionic portion of the surfactant package for emulsion polymerization.(11) Thus, by utilizing the knowledge from this previous research on new hydrophobes and the narrow-range ethoxylation technology, anionic analogs should be possible. Currently, the industrial standard anionic surfactant for emulsion polymerization is an NPE 3-mole ethoxylate sulfate. This APEO ether sulfate is very versatile and resilient to a wide variety of processing conditions in multiple types of emulsion polymerizations. One reason for the anionic surfactant’s success is the lack of free nonylphenol sulfate. Therefore, to best match these characteristics based on the previous knowledge, a narrow-range (NR) TDA or FT-OXO ether sulfate (Figure 3) should manage to satisfy these requirements.
In order to better demonstrate the performance of the sulfates discussed above as anionic APEO-free surfactants, a study was undertaken to employ a standard emulsion polymerization. The following sections discuss the surfactants evaluated, the polymerization and the analysis methods employed.
Sulfation

|
| Figure 4 Click to enlarge |
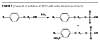
In order to examine the effect of APEO anionic surfactant alternatives on emulsion polymerization, the model monomers need to be synthesized. With APEOs, the sulfation is accomplished by reacting the nonylphenol ethoxylate with sulfamic acid, which is a mild sulfation agent.(12) This sulfation reaction is done in a batch process. The sulfamic acid preferentially reacts with a terminal hydroxyl group to form an ammonium sulfate salt (Figure 4).
Unfortunately, there are some drawbacks to sulfation with sulfamic acid. Using this batch process and a more costly sulfation agent increases the costs for the production for APEO-based ether sulfates. Furthermore, only the ammonium salt is produced by this technique, and the ammonium salt can be less stable due the volatilization of ammonia over time.
|
| Figure 5 Click to enlarge |
A less expensive sulfation process is called thin film sulfation using air/SO3. In this case, the sulfation agent is sulfur trioxide produced directly on demand. Pure sulfur is burned in very dry air to make sulfur dioxide, which is then oxidized over a V2O5 catalyst to form SO3. This process is continuous, efficient, economical and widely practiced in the surfactant industry,(13) but, unfortunately, it cannot be used for the sulfation of APEOs. That’s because the sulfur trioxide, a very strong sulfation agent, will sulfonate the phenol ring as well as sulfate the intended hydroxyl group (Figure 5).(14)
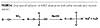
|
| Figure 6 Click to enlarge |
This leads to poorer surfactant properties, resulting in unstable emulsions; however, the alcohol ethoxylate alternatives to APEO do not have the phenol ring. For the surfactant industry, these are sulfated using the more efficient and less expensive thin film air/SO3 sulfation process (Figure 6). With this sulfation technique the alcohol ethoxylate sulfuric acid ester can be neutralized with any desired base, in contrast to the sulfamic acid technique, which yields only the ammonium salt. (12-14)
Experimental
For this study, an ammonium salt of nonylphenol ether sulfate with 4 moles of ethylene oxide was employed as the standard APEO ether sulfate for the anionic in the forthcoming emulsion polymerization. This material was obtained from an existing supply. For the APEO-free alternative ether sulfate, two isotridecyl-based ethoxylates were chosen. One was based on the standard base catalysis to form the broad range ethoxylate (BRE), and the other used the narrow-range technology (NRE).(15,16) These proceeded to sulfation.

|
| Table 2 Click to enlarge |
Sulfation
For the APEO alternatives, a laboratory thin film sulfation unit sulfated the ethoxylates at a SO3/ethoxylate mole ratio of 1.01. The resulting acids were neutralized in a separate reactor in a continuous fashion with sodium hydroxide to form a ~25% active solution. The NPE ether sulfate standard was obtained from a vendor and used “as is.”
Latex Production
This study employed semi-continuous emulsion polymerization to produce all-acrylic latexes. Table 3 outlines the formulation utilized for this examination. The theoretical solids are 55.01% and 54.72% after the post add.

|
| Table 3 Click to enlarge |
Polymerization
The polymerization reaction kettle was filled with the prescribed amount of water, purged with nitrogen and heated to 80 °C using a water bath. The nitrogen blanket was maintained throughout the entire polymerization. The monomer mixture and aqueous mixture were prepared separately. Each of the components for the respective mixtures were added slowly together and blended thoroughly.
The initiator solution was prepared by dissolving potassium persulfate in water. The monomer and aqueous mixtures were fed separately but simultaneously into the reaction vessel over a 4 h period while the initiator solution was added over a 4 h and 10 min period. The polymerization temperature was maintained at 80 °C throughout the addition time and for an additional 2 h to ensure complete conversion. The reaction kettle was cooled to ambient temperature over a 2 h period. Next, the post add, which is a linear 8-10 alkoxylate from Rhodia (referred to as ANTATROX BL225), and water were placed into the emulsion to increase the stability of the final latex formulation. Finally, the pH was adjusted to 8 using ammonium hydroxide to ensure stability of the anionic surfactant.
The total surfactant package for the emulsion polymerization included only anionic surfactant, and in this formulation no nonionic surfactants were examined. This allowed for the only stability effects for the polymerization to be due to the anionic surfactant being examined.

|
| Table 4 Click to enlarge |
Analytical Analysis
Several analytical methods were used to assess the composition, quality and properties of the reagents and final latex.
Particle Size Analysis: The latex particle size and distribution were determined using dynamic light scattering on a Microtrac UPA 250 analyzer. The analysis was performed using the procedure outlined by the manufacturer.
Wet Coagulum and Percent Conversion: For each latex, the percent conversion and wet coagulum were determined gravimetrically.
Percent Solids: Percent solids was determined for each latex utilizing ASTM D 2369.
Percent Free Alcohol: Internal gas chromatographic method.
Percent Polyethylene Glycol (PEG): Internal liquid chromatographic method.
Melting Point: Internal method.
Results and Discussion

|
| Table 5 Click to enlarge |
Surfactant Composition
For the properties of the three sulfates see Table 4. The percent active is slightly low for the industrial standard, and the lower actives in the alternatives can be attributed to the laboratory scale reactor. Despite this, the properties of the APEO-free alternatives are close to that of the NPE ether sulfate.
Emulsion Properties
The resulting all-acrylic latex properties are outlined in Table 5. When comparing the APEO standard to the alternatives, all maintained high conversion and stability. Both the alternatives had slightly lower conversion, but this is probably a factor of the formula optimization for the APEO. When optimized the conversion is expected to be equivalent to that of the APEO. Despite this, the alternatives performed consistently as a drop in replacement for the NPE ether sulfate. Additionally, the APEO alternatives would not have problems that affect most APEO ether sulfates such as evaporation of ammonia, which yields the acid form of APEO ether sulfate and high odor.

|
| Table 6 Click to enlarge |
Interestingly, the alternative anionic surfactants did not seem to be influenced by the ethoxylation catalyst. One would have expected the NRE-based surfactant to outperform the BRE surfactant; however, when the starting ethoxylate materials are examined (Table 6), the two nonionic surfactants are very similar. The major difference is between the polyethylene glycol levels, which impacts the melting point dramatically. The NRE catalyst technology does not illustrate its full potential on these low-molecular-weight ethoxylates as compared to the high-molecular-weight ethoxylates from the previous nonionic surfactant emulsion study. There the NRE technology gave surfactants that had very different physical properties due to the lower polydispersity of ethoxymers. Here, the minor differences in the ethoxylates lead to only minor differences in the sulfate analogs. Therefore, the resultant sulfates should, and do, behave similarly to one another.
Conclusions
APEOs and their sulfate derivatives have been the backbone surfactants of the emulsion polymerization industry, yet perceived environmental concerns and industrial pressure have led companies to seek non-APEO alternatives for latex production. In this study, the APEO-free alternatives provided emulsification during the polymerization equal to the industrial standard, NPE ether sulfate, as a “drop in” replacement. The alternatives have additional benefits, such as not being ammonium salts, which could convert back to the acid form over time. Interestingly, the model emulsion study demonstrated little difference between the narrow-range ethoxylation (NRE) catalyst and the standard broad-range catalyst in the performance of the sulfate analog. This relates back to the original ethoxylates, which were very similar because the NRE catalyst full effect is not seen until higher levels of the ethoxylation.
Acknowledgements
We would like to acknowledge the University of Southern Mississippi for help in evaluating the surfactants.
This paper was presented at The Waterborne Symposium, New Orleans, LA, 2010. The symposium is sponsored by The Univesity of Southern Mississippi School of Polymers and High Performance Materials.

