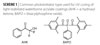
In recent years the field of waterborne UV-curable coatings has taken on greater significance as the coatings industry recognizes how it can help increase production efficiencies, lower volatile organic compounds (VOCs), and deliver high-end performance.(1,2) In previous papers, BASF described how various waterborne urethane acrylate dispersions could be photocured and also photostabilized.(3,4) We found a particularly effective strategy for photocuring was to use two or more photoinitiators.
|
| Scheme 1 Click to enlarge |
The individual photoinitiators target specific regions (surface and bottom) of the coating. Short-wavelength-absorbing photoinitiators were found to effectively cure the top coating surface. On the other hand, a long-wavelength-absorbing and photobleachable photoinitiator can be effective for deep through-curing. Examples of the common photobleachable, long-wavelength-absorbing photoinitiators are monoacylphosphine oxide (MAPO) and bisacylphosphine oxide (BAPO).
Photoinitiators that work well to cure the surface are aromatic α-hydroxy ketones (AHK) and mixtures of benzophenone/AHK.(5) To ensure good through-curing arylphosphine oxide photoinitiators were employed.(6) Deep through cure was particularly critical for coatings that contained UV-blocking materials (light stabilizers and/or pigments/fillers). Water-dispersible benzotriazole (BZT) and polar hindered amine light stabilizers (HALS) were found to be useful in these early studies.3 These light stabilizers, however, do not necessarily offer the best performance for low wash-out and high photopermanence.
|
| Scheme 2 Click to enlarge |
Since that time, newer high-performance waterborne UV-curable resins have been introduced into the marketplace,(7) and these in turn place more stringent requirements on the photoinitiator and light stabilizer packages. Further, the higher durability requirements often are not met with traditional light stabilizer packages.
Part of solving the light stabilizer problem was trying to get high-performance and hydrophobic additives into the resin system. A new way to overcome this barrier was recently introduced to the coatings industry.(8,9) The solution was to use an encapsulation technology for the high-performance additives. The so-called Novel Encapsulated Additive Technology (NEAT) was found to provide a way to easily incorporate the materials in aqueous paints without using co-solvents and without requiring high-energy dispersion equipment.
The goal of this article is to explore the use of NEAT additives for a UV-curable waterborne resin, which yields a photostable coating. We found that stability, control over color and increased weatherability were achieved using NEAT light stabilizers with liquid-based photoinitiators.
Experimental
Materials
Ethylene glycol monobutylether (EB) from Acros Organics was used as an additive co-solvent for selected photoinitiators and/or light stabilizers.

|
| Table 1 Click to enlarge |
BASF photoinitiators were used as is, which included the α-hydroxyketones (AHK), mixtures of benzophenone and AHK, and mono and bisacylphosphine oxides. The AHK photoinitiators used were: Irgacure® 2959,(12) which is a solid, water-soluble AHK, blended Irgacure 184 with EB, and an experimental liquid AHK (LAHK). The Irgacure 500 was a 1:1 liquid blend of benzophenone and Irgacure 184. The acylphosphine oxide photoinitiators included the monoacylphosphine oxide (MAPO) and the bisacylphosphine oxide (BAPO). The BAPO examined was in three forms: dispersion, liquid mixture with either MAPO or AHK. The Irgacure 819-DW is a water dispersion of BAPO. The liquid Irgacure 2022 is a blend of AHK/BAPO.(6) The Irgacure 2100 is a liquid blend of BAPO/MAPO.(6)
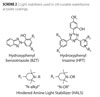
The BASF light stabilizers used consisted of UV absorbers (UVAs) and hindered amine light stabilizers (HALS). Tinuvin® 1130 (UVA-BZT) and Tinuvin 292 (HALS-292) were used as is. The Tinuvin 123-DW (HALS 123-DW) is a nonpolar HALS (Tinuvin 123), which uses the NEAT technology to render it 30% active in an aqueous solution. The Tinuvin 400-DW (UVA 400-DW) and Tinuvin 477-DW (UVA 477-DW) are both hydroxyphenyl triazene UV absorbers. The UVA 400-DW and UVA 477-DW are both 20% active in an aqueous solution.
Polyurethane Dispersion (PUD) Formulation
BASF Laromer® 8949 was selected as the resin for the UV-curable aliphatic polyurethane dispersion. Laromer 8949, 38-42% active,(12) is a waterborne, energy-curable aliphatic urethane dispersion with physical drying properties. This high-performance resin is intended for the production of coatings for paper, wood, wood-based materials, plastic and printing inks.(7)
A low-gloss, fast-curing formulation using Laromer 8949 as the base resin is given in Table 1. This formulation, hereafter referred to as PUD, is suitable as a coating for wood and/or for plastic.
Photoinitiators were added by simple mixing to the formulation to make the resin UV curable. The light stabilizers were also added by simple mixing, and they were used to determine their effect on photodurability, color and photoresponse.
Formulation Stability Testing
The formulations were prepared containing various additives and stored at 23 ºC. The samples were examined for any change in appearance over time, such as the development of phase separation, thickening and/or gelation.
Photocuring and Characterization
Samples were applied using a Bird® bar by Bird Film Applicators, Inc. onto white aluminum panels, dried at room temperature for 5 to 10 minutes, then placed in a convection oven at ~53 ºC for 10 minutes. The dried specimens were subsequently photo-cured in air at ambient temperature with a Fusion UV 600 watt gallium doped “V” lamp followed by an iron doped “D” lamp at various energy levels. The typical line speed was 20 feet per minute. A single pass under the lamps corresponded to a UVA energy of 3.8 J/cm².
A radiometer UV Power Map® from EIT Inc. was used to measure UV light on the conveyer belt. The diode detector is sensitive to the following regions: UVV (395-445 nm), UVA (320-390 nm), UVB (280-320 nm), UVC (250-260 nm).
Xenon Accelerated Weathering
The Xenon WOM measurements were performed with a Weather-Ometer® from Atlas Electric Devices Company, following the SAE J1960 test protocol. To this end the xenon arc burner was equipped with a quartz inner filter and a “Type S” borosilicate outer filter. A Cam #180 also was installed. The light cycle used had an irradiance of 0.55 W/m² @ 340 nm, with black panel temperature at 70 ºC, wet bulb depression at 12 ºC and conditioning water at 45 ºC; dark cycle used: black panel temperature at 38 ºC, conditioning water at 40 ºC.
The CAM #180 installed provided 120 minutes of light and 60 minutes of dark in the following cycle: 40 minutes of light followed by 20 minutes of light and front specimen spray, followed by 60 minutes of light, followed by 60 minutes of dark with back rack spray and repeating. The coated sample specimens were visually inspected after exposure and characterized for changes in color and/or solvent resistance.
FTIR Double Bond Conversion
A Nicolet™ Avatar™ 370 DTGS spectrophotometer by Thermo Electron Corporation was used to obtain the FTIR/ATR spectra. The spectrophotometer was equipped with a diamond crystal and a smart orbit accessory. Data acquisition used a resolution of 4 cm-1 and data spacing of 1.929 cm-1; each spectrum was an average of 32 scans, taken from 4000 cm-1 to 400 cm-1 and referenced against an air background.
The reported percent double bond conversion (% conv) was determined from ATR-FTIR spectra. The double bond conversion is expressed as % conv = 100*(Initial-Final)/Initial, where Initial and Final observables are integral ratios of (acrylate absorbance integral at 815 cm-1)/(carbonyl absorbance integral at 1704 cm-1). ATR-FTIR spectra were obtained from both top and bottom sides of the coating.
Solvent Resistance
Solvent resistance, using methyl ethyl ketone (MEK) is a well-known method to determine cure. Double rubs are performed on the coating surface with a cloth soaked with MEK solvent.10
Coloristics

|
| Scheme 3 Click to enlarge |
Colorimetric measurements were performed with a Minolta® Spectrometer CM 3600d by Konica Minolta Sensing, Inc. The color values were specifically quantified by determining the CIE L*a*b* system, following ASTM E 308 methods.(11) A higher positive number for the b* value indicates a stronger yellow color.
Results and Discussion
The PUD formulation used the high-performing Laromer LR 8949, which is a waterborne, energy-curable aliphatic polyurethane dispersion for coatings for wood, wood-based materials, paper, plastic and printing inks. A generic structure of the resin is shown in Scheme 3.
Coatings based on the PUD formulation were tack-free after physical drying (evaporation of the water) and therefore are well-suited for three-dimensional substrates. After curing with UV light, the cured films show excellent resistance to water and chemicals (such as MEK), and are also very scratch- and block-resistant.
The methodology used to optimize the formulation with respect to selecting the photoinitiator and light stabilizer packages divided the screening work into three parts: 1) formulation stability, 2) photocure response, and 3) accelerated weathering.

|
| Table 2 Click to enlarge |
Effect on Solution Stability
As a first step in additive selection, it is necessary to determine their effect on stability of the waterborne formulation. Since this is a UV-curable PUD formulation, we needed to assess the stability of the individual photoinitiators (which are needed for curing), the light stabilizers (which are needed for photostabilization after curing and/or color management) as well as their combination.

|
| Table 3 Click to enlarge |
It was found that the photoinitiators commonly used for waterborne coating(2,4) systems showed excellent stability characteristics (Table 2). For example, good stability (>140 days) was observed for the dispersed BAPO (Irgacure 819-DW), for Irgacure 500 as well as for the liquid AHKs (LAHK and Irgacure 184/EB).
The solution stability with the PUD was more greatly affected by use of the light stabilizers. As given in Table 3, the combination UVA-BZT/HALS-292 proved unusable, because the shelf life stability was less than 1 day. In an effort to see if a small amount of co-solvent could overcome some of the stability issue, we co-mixed EB with the UVA-BZT/HALS-292. This resulted in an improved shelf life, but the stability still remained too low to be useful. In contrast, the NEAT products were especially good, and the shelf life was up to greater than 90 days.
Following the early work,(4) we initially examined the use of Irgacure 819-DW in combination with the light stabilizers

|
| Table 4 Click to enlarge |
of the NEAT (Tinuvin DW) type. The results in Table 4 show without exception low stability (<30 days) resulted for all formulations containing Irgacure 819-DW. The stability was as low as only 10-15 days for formulations with higher Irgacure 819-DW content. The results indicate that for this particular PUD formulation the Irgacure 819-DW is not a good option.
Effect on Photocure Response
The UV-cure profile can be assessed by a number of ways, such as spectroscopically and/or through physical testing such as tensile analysis or solvent resistance. In this study we examined the double bond conversion and solvent resistance.

|
| Figure 1 Click to enlarge |
FTIR analysis was used to determine the degree of conversion (measurement of the acrylate double bond). The results of how the UV exposure affects the conversion are shown graphically in Figures 2 and 3. From the FTIR analysis it was found that at the surface the double bond conversion was at best 60%. In contrast, excellent double bond conversion (90%) was seen for the bottom side of the coating.

|
| Figure 2 Click to enlarge |
As shown in Figure 4, the exposure to UV lamps caused, in some cases, an increase in color. Without light stabilizers the coating becomes increasingly more yellow (Db*>0) with increasing UV lamp exposure. Adding in the LS reduces the effect. Thus, without a LS package, the b* increases on all samples. With the LS the color is more stable. Using the Tinuvin 477-DW as the light stabilizer, we see that the coatings have a much higher initial b* (which is expected since the Tinuvin 477 is a super red shifted). The best results were seen with the use of Tinuvin 400-DW/Tinuvin 123-DW.

|
| Figure 3 Click to enlarge |
The Tinuvin 477-DW light stabilizer blocks light up to 400 nm. Indeed, it is the most red shifted hydroxyphenyltriazene that is commercially available. Its ability to block light is exceedingly good. For thin coatings (<1.5 mil) the long wavelength absorption does not cause a problem in giving the coating a yellow colored appearance. At a higher thickness, i.e., 2 mil, it can add color to the coating, which may be unacceptable. As shown in Figure 4, the coatings containing Tinuvin 400-DW/Tinuvin 123-DW have a b* of approximately 7 to 8. Thus, for color-sensitive applications the preferred UV blocker would be Tinuvin 400-DW.

|
| Figure 4 Click to enlarge |
A further advantage in using Tinuvin 400-DW in a UV-curable system is that the acylphosphine oxide photoinitiators are easier to photo-excite, since the UV blocking is basically limited to <375 nm and these photoinitiators absorb at longer wavelengths. This condition allows good through curing (see Figure 3).
The question now is at what light exposure is the coating considered well cured? As summarized in Table 5, it was found that a single pass under the lamps at 20 fpm (380 mJ/cm2) gave coatings with very high solvent resistance. From the MEK resistance test it was found that unexposed samples had poor solvent resistance (MEK double rubs <2). After a single pass under the lamps all the coatings had a MEK double rub >100, which indicated excellent cure.

|
| Table 5 Click to enlarge |
Based on these results we used a single pass exposure to prepare samples for accelerated weathering. We used the light stabilizer package consisting of Tinuvin 400-DW and Tinuvin 123-DW.
Effect on Accelerated Weathering
As given in Table 5, coatings maintained their solvent resistance properties even up to 1200 hours Xenon WOM exposure. The question is whether their color also could be stabilized. As given in Figure 5, the light stabilizer package (which consisted of 2% Tinuvin 400-DW and 1% Tinuvin 123-DW) was critical in maintaining color.
Indeed, as shown in the right side of Figure 5, with light stabilizers the Db* was within 0.5 units over 1000 hours Xenon WOM. We also saw a general decrease in color when the light stabilizer package was used.

|
| Figure 5 Click to enlarge |
In contrast, the Db* shifted dramatically without light stabilizers (see left side of Figure 5). For example, during the first 500 hours of exposure the Db* increased (from 0.5 to 1.5 units). In subsequent exposure the Db* actually decreased (approximately 0.5 units). The lowest color without a light stabilizer package was with the photoinitiator Irgacure 184/EB (1:1).
Conclusions
The fast, UV-curable, water-based PUD resin exhibited very high solvent resistance and durability. It was found that the NEAT was necessary for introducing high-performance light stabilizers into the UV-curable PUD formulation. The best overall performance was obtained using the NEAT light stabilizer products and liquid photoinitiators based on acylphosphine oxide. The optimal combination of light stabilizers (UV absorber 1% Tinuvin 400-DW, and HALS 2% Tinuvin 123-DW) with photoinitiators (0.2% Irgacure 2100 plus 1.2% Irgacure 184/EB) proved crucial for formulation stability and fast photospeed curing. They were also keys to maintain low color even under accelerated weathering.
The outlook for the future in using this eco-friendly technology remains bright. We expect this formulation knowledge can be successfully applied to even more-demanding applications, such as ultra-thin protective coatings for high-end applications.
This paper was presented at the RadTech 2010 Technology Expo and Conference, Baltimore, MD, www.radtech.org.





Report Abusive Comment