Nanotechnology

|
| Figure 1 Click to enlarge |
Simply stated, a nanometer is a metric unit of length equal to one billionth of a meter. Nanotechnology is the study of the controlling parameters of materials in atomic and molecular scale. Generally, nanotechnology deals with structures of 100 nanometers or less in at least one dimension, and involves developing materials or devices of that size.
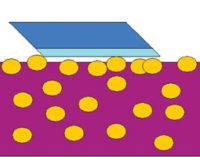
Nanoparticles are just like any other solid inorganic or organic particle that we try to incorporate into a resin matrix. If we just mix the particle into the solution, it will sink to the bottom because the solid particle has no compatible surface with the solution. It is similar to trying to float rocks on the surface of a lake. The rock is much denser than the water, and the water doesn’t have the required matrix to hold the rock at the surface.
Figure 1 represents the dispersion of nanoparticles into liquid media with a proper wetting and dispersing additive. This is imperative for the functionality of these solid particles in today’s coatings systems. More energy must be used to disperse nanoparticles than micro pigments because of their small size and high surface energies.

|
| Figure 2 Click to enlarge |
Once these solid nanoparticles are stabilized by steric hindrance or charge stabilization, they are easily mixed (1000 rpm for 2 min) into any compatible resin, clear or pigmented coating. Figure 2 shows the structures of controlled flocculation and deflocculation.
Dispersed Nanoparticles Enhance the Coating's Life Cycle
Additives containing alumina, silica, ceria and zinc oxide nanoparticles can provide improved wear and UV resistance for solvent, waterborne and UV coatings. These nanoparticles are predispersed in different media and easily incorporated with low shear into aqueous, solventborne, or solvent-free UV-curing systems. They work by setting up a network within the resin matrix to make the coating more resistant to damage from daily marring and scratching.
Dispersed nanoparticles (alumina, zinc oxide and silica) can also improve corrosion resistance. This is accomplished through cathodic protection. This is a technique used to inhibit corrosion of buried or immersed structures by supplying an electrical charge that suppresses the electrochemical reaction. If correctly applied, corrosion can be stopped completely. In its simplest form, it is achieved by attaching a sacrificial anode, thus making the iron or steel the cathode in the cell formed. Due to the large surface area, nanoparticles are often more efficient than micro particles, resulting in lower concentration necessary to obtain good results.
Surfaces exposed to direct sunlight usually suffer from solar UV radiation that destroys organic compounds such as binders, polymers, organic pigments and substrates. Zinc oxide and cerium oxide nanoparticles can stop solar degradation by absorption of the UV radiation. They absorb UV light and transfer it into vibration and heat. By transferring the UV light, the level of energy is lowered and does not destroy the polymer matrix. This absorption method also protects the substrate by not allowing UV radiation to penetrate through the coating. Different from organic UV absorbers, inorganic UV absorbers do not suffer from UV light, resulting in long-term protection.
Typically, low dosages of nanoparticles, 0.5-2.0%, provide significant and long-term scratch, mar, wear, corrosion and UV resistance without adversely affecting gloss, color, clarity or other physical properties of the coatings.
|
|
Figure 3 Click to enlarge |
Nano Stabilization
BYK has practiced the concept of pigment stabilization since the 1870s, and has developed over 300 different types of wetting and dispersing additives over the last 50 years to meet industry needs. From the simple cook process to controlled polymerization, we have developed additives and unique processes for stabilizing different types of nanoparticles.
Once these nanoparticles are stabilized with BYK’s propriety boundary phase technology, they can be predispersed in different media and easily incorporated with low shear into aqueous, solventborne or solvent-free UV-curing systems, or compounded into plastics.
Initially, I theorized that these nanoparticles were being totally coated (encapsulated) by the wetting, dispersing or silicone additive, similar to what happens to micronized pigment particles (creating a + charge around the pigment surface).
In 2006, I proposed that the nanoparticles form structural lines of particles similar to a string or strand of pearls throughout the clear resin matrix, thus forming a structure that strengthens the resin matrix. This string structure also gives the resin greater elasticity to endure the scratch and marring, rather than making the resin harder. This theory was confirmed in 2010 by the work of Matt Lane at Sandia National Laboratories, Albuquerque, NM (Figure 3).(1) This added credence to our work with dispersing nanoparticles and how these predispersed nanoparticles function within the coating.

|
| Figure 4 Click to enlarge |
Nanoparticles must be uniformly distributed throughout the coating to provide the resin or coating with a continuous, solid, protective network layer of Al2O3 or SiO2. This is the unique nano advantage. As a coating is worn away, additional layers of nanoparticles remain to resist continued scratching. The nanoparticles also form a unique elastomeric structure within the coating. This structure resists objects from entering the surface of the coating and promotes the reflow of the coating, thus preventing severe damage to the coating (Figure 4).
The nano silica and alumina particles not only offer scratch resistance but can provide greater wear resistance, better adhesion and anti-staining because the nanoparticles create a denser, more compact film structure.

|
| Figure 5 Click to enlarge |
Under normal conditions we consider a scratch and mar coating failure to occur when a coating loses 10% of it’s original gloss. The control had a starting gloss at 85º, while the nanocoating started at 90º gloss. Figure 5 shows that the control lost over 10% of its gloss after 150 cycles. The control continued to have the surface damaged until the gloss was below 20º after 800 cycles. This gives an indication that the control is not good for long-term scratch and mar resistance
The same coating with the addition of 2% silica in TPGDA did not lose 10% of its gloss until 1100 cycles. The nanocoating continued to have a gloss above 70º at 2000 cycles. This shows that the nanocoating was ten times better in scratch and mar resistance than the control. This is a good example of nanoparticles being able to improve the life cycle of the coating.
Nanoparticles Improve Corrosion Resistance
Nanoparticles are uniformly distributed throughout the coating, thus providing the resin or coating with a continuous, solid, protective network layer of Al2O3 or SiO2. The nanoparticles also form a unique elastomeric structure within the coating, and create a denser, more compact film structure. Cathodic protection is a technique used to inhibit corrosion by supplying an electrical charge that suppresses the electrochemical reaction. If correctly applied, corrosion can be stopped completely. In its simplest form it is achieved by attaching a sacrificial anode, thus making the iron, steel or aluminum the cathode in the cell formed. Nanoparticles have a large amount of electrons -on their surface, which can create this sacrificial anode. This again is one of my theories of the functioning of nanoparticles for corrosion resistance.

|
| Figure 6 Click to enlarge |
Figure 6 shows that under 40X magnification, the creepage of the rust on the control panel was at least five times larger than the coating with the nanoparticles added. When you look at the magnification of the nanocoating, you can see there is almost no creepage after 580 h of salt spray. This was an automotive OEM clearcoat coating over ED coating on steel. The nanoparticles were post added to the formula and sprayed. This shows that nanoparticles can add a longer life to the automotive OEM coating.
Figure 7 shows a water-reducible, direct-to-metal primer. The primer with no corrosion control additives showed complete failure after 260 h of salt spray. The standard corrosion package at 500 h salt spray showed heavy rust, and when scratched showed a 2 millimeter under creepage. The nanocoating showed almost no rust after 500 h, and when scratched had no under creepage. Helping to slow down corrosion is another way nanoparticles increase the life cycle of a coating.

|
| Figure 7 Click to enlarge |
Amongst inorganic UV absorbers, zinc oxide and cerium oxide stand out with almost complete adsorption of UV-A, B and C. Reduction of particle size from micron to nanoscale enables the formulator to formulate clear coatings or adhesives that can both enhance the appearance of substrates as well as provide long-term protection for wood, metal and plastic substrates against UV degradation. Inorganic UV absorbers can outperform chemical UV absorbers because they are more permanent within the resin or coating, whereas the chemical UV absorbers gradually degrade over time within the resin (Figure 8).
Safety of Nanodispersions
Questions regarding the impact of released nanoparticles from use and wear remain. BYK worked with the German Government and NIST to evaluate nanoparticles in coatings. Two studies have been completed. The first study provided results by Tabor abrader. The article, titled Method for the Characterization of the Abrasion-Induced Nanoparticle Release into Air from Surface Coatings, was published September 30, 2008, in Elsevier Aerosol Science. The study concluded, “During the abrasion test no significant release of particle concentrations (<100 nm) was generated in the aerosol flow. Therefore the nanoparticles are probably embedded in the generated wear. This could be confirmed with SEM, TEM and EDX. The nanoparticles were clearly visible and embedded in the resin and showed the characteristic morphology of the zinc oxide.”

|
| Figure 8 Click to enlarge |
The second study provided results by sanding. The article, titled Characterization of Nanoparticle Release from Surface Coatings by Simulation of a Sanding Process, was published August 2, 2010, via the internet http://annhyg.oxfordjournals.org. “This conclusion agrees with the first study, that during abrasion test no significant release of particle concentrations (<100 nm) was generated.”
The studies were conducted by the Research Group Mechanical Process Engineering, Institute of Process Engineering and Environmental Technology, Techhnische Universitat Dresden, by Daniel Gohler, Michael Stintz, Lars Hillemann and Manuel Vorbau.
Conclusion
Based on our studies, we conclude that predispersions of nanoparticles:
- Are easy to mix into coatings;
- Provide homogeneous distribution of nanoparticles in the coating film;
- Prevent coating damage by innovative, immediate reflow effect;
- Absorb the impacting energy like a shock absorber;
- Do not increase the brittleness or reduce chemical resistance;
- Are suitable for all systems, from low to high polarity.
This is why we believe predispersed nanoparticles can be very beneficial to the life cycle of coatings. Nanoparticles have a lot to offer to the coatings industry. This is new technology, and we can truly say, “We have only scratched the surface.” There is much more still undiscovered.
This paper was presented at the 38th Annual Waterborne Symposium, February 2011, in New Orleans.
For more information, contact Robert McMullin at Robert.mcmullin@altana.com.
Appendix
Testing Procedures
The incorporation of additives into the coating formulations was as follows:
- The additives were post added using a Dispermat CV with a 40 mm Cowles blade at 600 rpm for 2 min. In case of combination of wax and nano, the wax was added first at 600 rpm for 2 min and then nano at 600 rpm for 2 min.
- Additives dosage
Nano Al2O3 or SiO2, wax, acrylates and silicones
All additives dosages mentioned are based on total formula weight (tfw).
Application
Substrate: Masonite board (for abrasion test), Leneta chart (for scratch test), hot rolled steel, cold rolled steel and aluminum panels (for corrosion testing).
Application tool: wire-wound bar, draw down bars and low-pressure, high-volume spray.
Coats: 3 on Masonite board and 1 on Leneta chart, 1 coat on metal panels.
Thickness: 4 mil wet of each coat. Thickness of dry film depends of percent solids of coating.
Dry time: 2 h between each coat and at least 48 h after final coat for corrosion testing seven days before scribing and placing into salt spray.
Sanding: after 2 h drying of 1st and 2nd coat, with a very fine sandpaper (220 grit) to ensure proper adhesion of additional coats.
Scratch Test
Each sample drawdown was evaluated for scratch resistance with 20 double rubs using 9 micron polishing paper pads on abrasion tester (dry) from BYK-Gardner. Gloss (20º) (60º) was measured (statistical average of 5 readings) using micro-TRI-gloss from BYK-Gardner before and after scratching the panels. Percent gloss retention was also calculated as follows
% Gloss Retention = 100 x Gloss (60º) of scratched area
Original gloss (60º)
% Gloss Retention = 100 x Gloss (20º) of scratched area
Original gloss (20º)
Testing for scratch covers both areas of mar and scratch
Abrasion Test
ISO 9352 or ASTM D 1044.
Taber Abraser (from TABER Industries)
CS-10 abrading wheel, 1000 gm load, 100, 250, 500, 750, and 1000 cycles
Pendulum Hardness Test – König
After allowing the coating to dry for 8 h, and 48 h, a König pendulum hardness test was run. More objective than using pencil hardness testing.
Light Microscopy
A light microscopic image of each sample coating was taken after scratch test. Images were taken by MOTIC lens at 40X magnification.




Report Abusive Comment