The ever-increasing attention paid to environmental protection has produced a lot of legislation aimed at reducing emissions from industrial activities, particularly VOCs used as diluents in coatings1. Besides the safety and protection regulations placed on plants, emissions from VOCs are also being substantially reduced through the development and use of new low-solvent and solvent-free coatings, such as high-solids paints, waterborne coatings and powder coatings. Regarding waterborne coatings, many efforts have been made to produce alkyd resins able to provide dispersion paints with less organic solvent (less than 10 to 15%) for the finishing of metal surfaces.
Waterborne alkyd resins2 are polymeric derivatives with a high acid number (AN greater than 50) which provides the required water solubility upon neutralization and salt formation with aliphatic amines, usually trimethylamine for air-drying alkyd resins, or dimethylethanolamine for oven-drying alkyd resins3. This method is mainly applied with medium- (40 to 60% oil content) and short- (less than 40% oil content) oil resins, modified with drying and semidrying oils/fatty acids. Since the short-oil resins can be crosslinked by reaction with oligomeric aliphatic or aromatic isocyanates4, it is thus possible to produce two-pack systems to be mixed immediately before application that quickly give rise to crosslinking. Optimal paint films are generally formed when the NCO/OH ratio is equal to 1.
However, it may be advantageous to deviate slightly from this ratio. When the ratio is less than 1 (undercrosslinking), some of the OH moieties remain unreacted and the produced coating has a good flexibility and good adhesion to the substrate, but low resistance to solvents and chemicals. If the ratio is greater than 1 (overcrosslinking), the coating displays longer drying time, enhanced hardness and improved chemical resistance, but lower adhesion to the substrate. The main areas of application of these coatings include transportation, industrial equipment, machinery, furniture and the surface treatment of plastics.

Isocyanate as a crosslinking agent
In addition to the above-mentioned properties, the use of isocyanate as a crosslinking agent for the resin may also allow the content of the drying oil to be reduced. This could potentially improve the stability of the coating when exposed to oxygen and sunlight. To check this possibility, a series of coatings were characterized in terms of gloss and weather resistance. The coatings were all formulated from short-oil alkyd resins and differed in composition only by type or amount of the oil/fatty acid component. This component ranged from drying (with a high content of unsaturated fatty acids), to semidrying (medium content), to nondrying oils (saturated fatty acids).
Synthesis of alkyd resins
The alkyd resins have been prepared from trimethylolpropane, phthalic and trimellitic anhydride as polyfunctional monomers, in the presence of benzoic acid as monofunctional reactant and of the oil/fatty acid component (Table 1). Stoichiometric ratios and reaction conditions were regulated to obtain a final acid number (AN) value in the range of 52 to 55 and a content of free hydroxy groups of 3 to 5% to achieve, respectively, both a satisfactory water solubility after treatment with aliphatic amine, and an adequate crosslinking degree upon reaction with isocyanate. A molar ratio NCO/OH equal to 1.5 was considered the most suitable for a satisfactory final behavior of the coating.Benzoic acid (4 to 6%), phthalic anhydride (24 to 26%), oil/fatty acid (28 to 30%) and trimethylolpropane (30 to 32%) in xylene (2 to 4%) were allowed to react at 225°C under a nitrogen atmosphere until the acid number of the mixture reached the value of 21. Then, trimellitic anhydride (5 to 7%) was added and the reaction kept at a temperature of 170°C until a final acid number of 52 to 55 was reached. After cooling at room temperature, the material was diluted to 80% in propyleneglycol dimethyl ether and submitted to a series of characterizations (Table 1).
Table 2 reports the weight content of fatty acids for each type of oil/fatty acid employed.

Coating preparation and application
The coating was prepared by stirring the resin (30 to 32%) for 10 minutes with water (40 to 42%) and 2-amino-2-methylpropane (AMP) so as to obtain a constant pH value of 7.6 to 7.8. Surface additives (1 to 2%), and a green pigment dispersion made of PY74, PG1, PB7 and PW68(25 to 27%) were also added at this stage. The formulation was then mixed with the crosslinking agent hexamethylene diisocyanate trimer (HDI-T) in a ratio of 20% by weight with respect to the alkyd resin, corresponding to a NCO/OH molar ratio of 1.5. Before application, the viscosity of the formulation was adjusted to 60 to 70 seconds flow time at 25°C in a Din 4 cup9. The pot life of all the paint samples was found to be within the two to three hours range. The final crosslinking of coated metal specimens (paint thickness 30 µm) was performed by accelerated curing in an oven at 70°C for 20 minutes.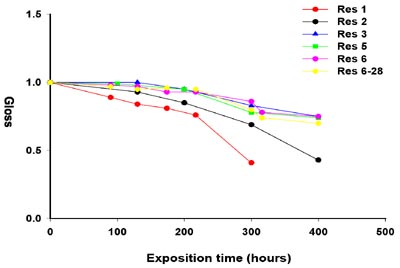
Coating weathering and characterization
Weathering was carried out in a light and water exposure apparatus (fluorescent UV-condensation type Q-Planet Lab Products) as depicted in Figure 1, operating in accordance with the ASTM G53-91 method. All specimens were submitted to repeated cycles of UV light irradiation with two coupled series of four fluorescent UV-B 40-watt lamps each (maximum emission wavelength at 313 nm) for four hours at 50°C followed by a water condensation step of four hours at 40°C according to the above-mentioned method.
After known time intervals of exposure, the specimen's gloss was checked at light incidence of both 60 degrees and 20 degrees10 on all the cured samples and compared to the initial one taken as reference by a Rhopoint Trigloss mod. 20/60/85 instrument. The presence of visible surface defects, such as cracks, blistering, etc., was also noticed.

Resins with nondrying fatty acids better
Resins 1 to 6 (Table 1) were treated to give the corresponding coatings, with the exception of Resin 4, which displayed an overly high Gardner viscosity to allow the intended application and characterization. This was probably a consequence of the fully saturated structure of the stearic acid present in the sample. In addition to crosslinking with 20% isocyanate, Resin 6 was also tested with 28% isocyanate (Resin 6-28). This compensated for the higher overall hydroxyl content due to the exclusive presence of ricinoleic acid and kept the established 1.5 molar ratio NCO/OH constant.After curing, the values of 60-degree gloss of the coated specimens were measured before the weathering process and were found to be quite high, between 90 and 95 (Figure 2). For this reason, the 20-degree gloss values were also measured, resulting in the range of 70 to 85. The initial values were all normalized to 100 and determined again at known time intervals up to 400 hours of weathering.
With the exception of sample Resin 1, which displayed an appreciable lowering of 60-degree gloss (Figure 2) soon after 100 hours of exposure to light and humidity, all the remaining samples appeared to substantially maintain their initial gloss after 200 hours exposure time. More pronounced differences among them become evident after that. More detailed information can be drawn from the curves representing the observations of gloss at 20 degrees light incidence (Figure 3). The coatings obtained from Resins 3, 5 and 6 displayed less loss of gloss after the same extent of weathering than Resins 1 and 2, which completely lost their gloss after 300 and 400 hours, respectively. Coatings prepared from Resins 3 and 5 were constituted from predominantly or totally saturated fatty acids (Table 2) with a very low iodine equivalent, whereas the coating derived from Resin 6, containing ricinoleic acid only, appears to be quite similar to the nondrying formulations Resin 3 and Resin 5, regardless of whether the crosslinking was performed with 20 or 28% of isocyanate. This behavior could be related to the presence of the hydroxyl group of ricinoleic acid, which is involved in the crosslinking reaction of the coating with isocyanate, thus improving the stability of Resin 6 to light and humidity11.
Tests on coatings with the addition of the surfactant Surfaron D6N (2 to 5%), a phosphoric acid alkyl ester, to improve the pigment dispersion, were also performed on samples Resin 2 and Resin 6-28. However, the presence of a wetting agent in the formulation proved to be detrimental with regard to weathering as the production of severe blistering12 over the coating surface was noticed for all the samples examined after only 90 hours of exposure to humidity (Figure 4). This is probably due to the enhanced water affinity imparted to the coating by the presence of the surfactant.

References
1. Council Directive 1999/13/EC, Off. Journ. of EC, L85/1, 29.3.1999.2. D. Stoye, W. Freitag, Editors, Paints, coatings and solvents, Wiley-VCH Weinheim (1998) p. 45-46.
3. R. Küchenmeister, Farbe & Lack (1972) 78, 550.
4. P. Deligny, N. Tuck, Resins for surface coatings, Volume II, Alkyds & Polyesters, Ed. PKT Oldring, London (2000).
5. EN ISO 3682-1998.
6. ASTM D 1545-89 (R-1993).
7. ASTM D 1725-62 (R-1996).
8. Colour Index, The Society of Dyers and Colourists Eds., Lund Humphries, Bradford and London (1971).
9. EN ISO 2431-1996.
10. ASTM D 523-89 (R-1994).
11. R. Lambourne Editor, Paint and surface coatings: theory and practice, Ellis Horwood Limited, Chichester (1987) p. 48.
ASTM D 714-87 (R-1994).
Sidebar: Results at a Glance
Waterborne short-oil alkyd resins containing drying, semidrying and nondrying oil/fatty acids were synthesized to obtain coatings with different stabilities to light and atmospheric agents.The aqueous pigmented coatings derived from the resins were crosslinked by reaction with isocyanate and submitted to accelerated weathering by exposure to repeated cycles of UV light irradiation and water condensation, and their degradation evaluated by gloss-loss measurements.
The results indicate that coatings derived from resins containing nondrying (saturated) fatty acids behave better, as expected, than those containing drying oils. However, the coating derived from the resin containing ricinoleic acid only as the fatty acid component, displayed a remarkable weathering stability, similar to the nondrying formulations, owing to the likely involvement of the fatty acid hydroxyl moiety to the crosslinking reaction with isocyanate.
Luigi Angiolini is professor of industrial chemistry at the Faculty of Industrial Chemistry of the University of Bologna, Italy, and Director of the Department of Industrial and Materials Chemistry. Dr. Valeria Grenci studied macromolecular chemistry at the University of Bologna. She is doing her Ph.D. thesis in industrial chemistry at the Department of Industrial and Materials Chemistry of Bologna. Dr. Elisabetta Salatelli has a degree in industrial chemistry from the University of Bologna. A version of this article first appeared in the June editon of the European Coatings Journal.

Report Abusive Comment