
Matting agents
The most common method for adjusting the gloss levels of coatings is via the use of specific extenders called matting agents. Select applications such as office furniture require matte coatings rather than high-gloss coatings because they reduce glare and hide minor surface defects and imperfections. Defense aircraft coatings also require extremely low gloss to avoid visual detection. Factors influencing the matting effect include the matting agent’s characteristics (particle size, number of particles, concentration, refractive index, oil absorption value), incorporation technique, dispersion level, coating thickness and binder system employed. Matting agents impart low gloss by generating a microrough surface (Figures 1 and 2) as the coating shrinks, due to loss of volatiles during drying and resin crosslinking.
Several different matting agents are available commercially.
Precipitated silica and silica gels are forms of synthetic silica
that are very effective matting agents due to their high
oil-absorption values (also results in thixotropy). Silicas that
are surface treated with wax offer enhanced suspension
properties. For waterborne coatings, the incorporation of
hydrated silicas eliminates the need for high-speed dispersion
and dusting problems, and facilitates gloss adjustment in the
final coating without foaming.

Extender particle shape also influences gloss levels. For instance, at equal loadings, wollastonite, an acicular (needle-like) extender, has been shown to be more effective at reducing gloss than spherical extenders, such as calcium carbonate and barium sulfate in powder coatings.1
Waxes are employed in coatings to alter surface characteristics, such as gloss, slip and abrasion resistance, and
include both natural waxes (e.g., bees wax, montan wax, carnauba wax) and synthetic waxes (e.g., micronized
polyethylene, polypropylene and polytetrafluoroethylene). During film formation, the waxes display a floating effect
and come to the surface. Film properties are related to the particle size, with larger particles providing greater
gloss reduction, while smaller particles impart better feel and smoothness. Some polyolefin waxes are used in
conjunction with silicas for enhanced suspension properties and improved surface feel.
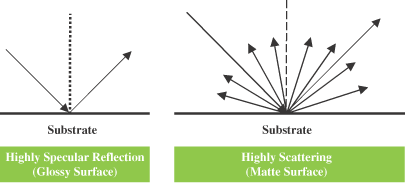
Glossy and Matte Surfaces
Metallic pigments
Metallic and pearlescent pigments are employed in coatings primarily for specific aesthetic effects. Metals employed as pigments include aluminum, zinc, gold bronze, nickel and stainless steel. Aluminum pigments (Figure 3) are manufactured by milling greater than 99% pure aluminum powder in the presence of a suitable lubricant (either stearic or oleic acid) and a suitable solvent. The choice of lubricant results in two different grades of aluminum pigments: leafing and nonleafing.Leafing grades involve the use of stearic acid as the lubricant, and result in aluminum flakes orienting themselves in a parallel and overlapping manner at the film surface to provide a solid, silvery appearance. The leafing phenomenon is influenced by variables such as surface tension, solvent evaporation, convection currents and coating viscosity. Leafing grades are used chiefly in trade sales and maintenance coatings.
Nonleafing grades contain oleic acid as the lubricant, and the aluminum flakes orient themselves throughout the coating film. Nonleafing grades generate aesthetically appealing finishes when used with colored pigments, and are used extensively in automotive coatings. Stabilized aluminum pigments are available for use in waterborne systems to prevent the inherent reactivity of aluminum with water.
The first generation aluminum pigments were thin flakes with irregular edges (“cornflake” pigments). Improved brightness and greater two-tone or flop effect (angle-dependent lightness) were achieved with the development of thicker and smooth-edged aluminum flakes (“silver dollar” pigments).
Even greater improvements in brightness and flop effect were achieved through the development of vacuum-metallized aluminum pigments produced via physical vapor deposition. Metallic pigments exhibit a combination of specular reflection from the metallic surface and scattering at the pigment edges or defects on the pigment surface. Vacuum-metallized pigments are significantly thinner than conventional aluminum pigments and have very smooth surfaces. Consequently, edge- and surface-scattering is minimized, and a highly reflective mirror-like effect is achieved with vacuum-metallized pigments.
Traditionally, metallic-finish powder coatings were produced by homogeneously dry-blending metallic pigments
with powder coatings. Dry-blend coatings are capable of producing quality finishes, but they have their limitations.
Differences between the metallic pigments and the powder coating, in terms of particle size, shape and density,
lead to stratification of the powder coating upon storage, and the phenomenon is further exacerbated by vibrations
or movements during handling and transportation. The resulting separation and accumulation of the metallic
pigments compromises the aesthetic appearance and raises safety concerns since metal agglomeration can lead
to short circuits within the powder delivery system.
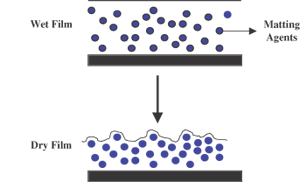
Matting Effect
Coated metallic pigments contain silica/ acrylic coating on the surface to assist them in acquiring the charge necessary for high transfer efficiencies during application and possess improved resistance to acids, alkalis and outdoor weathering. Spray characteristics are also enhanced because the specific gravity of coated aluminum flakes is intermediate to that of the uncoated pigment and powder coating resins. Eckart’s (Louisville, KY) Alucolor range of colored aluminum pigments contains lightfast and weatherproof organic pigments in the silica coating on the aluminum flakes and offers new colored effect pigments for use in powder coatings.
Shear forces in some coating application systems are capable of breaking and deforming aluminum flakes, and result in dulled metallic appearances. Circulation-resistant pigments, such as Silberline’s (Tamaqua, PA) Tufflake, are specifically engineered to withstand such mechanical forces and maintain the targeted appearance.
Zinc pigments are available in dust and flake form, and are used primarily for their excellent corrosion-resistance properties. Zinc flakes impart a bright metallic appearance and combine the intrinsic galvanic protection of zinc with the barrier protection of flake-shaped pigments.
Gold-bronze pigments are actually copper-zinc alloys. Four standard color options are available based on varying copper-zinc ratios: rich gold, rich pale gold, pale gold and copper. Eckart’s Dorolan silica-encapsulated gold-bronze pigments offer four more colors: English green, lemon, deep gold and fire red. Silica-encapsulated grades are recommended for powder coating applications.
Nickel pigments are available in powder or flake form. Due to their high electrical conductivity, nickel pigments are used in the manufacture of electrically conductive coatings for electromagnetic interference (EMI) and radio frequency interference (RFI) shielding requirements. Moreover, nickel pigments are corrosion-resistant and, being inert to water, will not generate hydrogen gas in waterborne coatings.
Stainless-steel pigments are manufactured from corrosion-resistant, austenitic 316L-grade stainless steel.
Stainless pigments possess excellent abrasion resistance and maintain their durability even in very aggressive
environments. Both leafing and nonleafing grades of pigments are available, and like nickel, stainless-steel
pigments are inert to water.

Pearlescent pigments
The search for pigments that duplicate nature’s creations, such as pearls, scarab beetles and butterflies, generated a series of pigments that exhibit varying levels of luster, color development and travel. The development of bismuth oxychloride platelet technology was followed by pearlescent pigments in the 1960s.Pearlescent pigments consist of thin, smooth layers of semitransparent, high-refractive-index (RI) materials, such as titanium dioxide and iron oxides on transparent flakes of low-RI materials such as mica (Figure 4). The high-RI materials strengthen the chroma by amplifying the angle-dependent interference colors of the low-RI flakes.
The pearlescent effect arises from the interference occurring between the light reflected from the oxide surface, and light reflected at the oxide-mica interface. The thickness and RI of the oxide layer determine the color of the reflected light and its intensity.
Mica platelets coated with titanium dioxide produce white or colored
pigments depending on the oxide layer thickness, while
iron-oxide-coated micas produce only colored pigments because of iron oxide’s inherent color. New coloristic
effects have been generated by replacing mica with other low-RI materials, such as silica (e.g., Savannah,
GA-based EMD Chemicals Inc.’s Colorstream) and alumina (e.g., EMD Chemicals Inc.’s Xirallic). The unique
luminous quality of pearlescent pigments creates interesting aesthetic effects when combined with conventional
absorption pigments.
Improved color travel (i.e., change in color with viewing angle) was achieved with the development of optically
variable pigments, such as Flex Products Inc.’s (Santa Rosa, CA) ChromaFlair, which contain layers of partially
reflective materials and weakly refracting materials around a reflective core.2 The core could be either a totally
reflecting, opaque material such as aluminum, or partially reflecting materials such as oxides or mica/oxides.
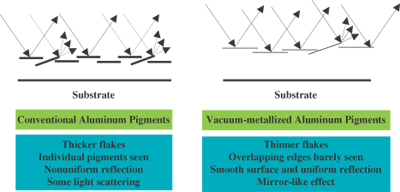
Aluminum Pigments
Cholesteric liquid crystal pigments, such as Helicone pigments, from
Wacker-Chemie Gmbh (Muenchen, Germany), create innovative,
optically variable color effects by reflecting certain spectral
components of the incident light and transmitting the remaining
components. Above their melting point, liquid crystals show
characteristics of both isotropic melt and ordered crystals, and
therefore, certain physical properties such as RI are angle-dependent.
Liquid crystals have several phases, of which the most common is the
nematic phase. The incorporation of chiral additives on to a nematic
liquid crystal leads to the formation of a cholesteric (also called
“twisted nematic”) structure.

Pearlescent and Optically Variable Pigments
When the pitch corresponds to the wavelengths of visible light, which is 400 to 700 nm, the reflected light is perceived as color. The reflected color can be varied depending on the amount of chiral additive. Moreover, the wavelength of the reflected light is angle-dependent with flatter viewing angles leading to shorter wavelengths, i.e., color changes as a function of the viewing angle.
Silberline’s Geometric Pigment consists of holographic flakes made from ultraclear polyester films that have been
holographically embossed and vacuum-metallized. The film is coated with an epoxy resin and cut into particulates.
Geometric Pigment is offered in various sizes and shapes, and is available in four colors: silver, gold, copper and
pewter. The holographic pigments can be used in powder coatings by dry blending.
Pigment developments
Modern coatings require pigments with high color strength, good dispersibility, good exterior durability and stability against heat, light and chemicals. Moreover, the trend toward environmentally friendly coatings, such as waterborne, powder and UV-curable coatings, has generated demand for pigments that are optimized for use in these systems.For instance, Sun Chemical’s (Cincinnati) phthalocyanine blue pigment Palomar Blue B-4828 is recommended for use in waterborne aluminum and mica automotive stylings. On the other hand, Sun Chemical’s Quindo brand of quinacridone pigments, Magenta RV-6883, Magenta RV-6858 and Violet RV-6956, are examples of pigments that were developed for use in high-solids systems, but may also be used in waterborne systems.
Clariant’s (Charlotte, NC) quinoxalinedione pigment, Hostaperm Yellow H5G, the benzimidazolone-dioxazine
pigment Hostaperm Blue R5R and the yellowish red thiazineindigo pigment, Novoperm® THI Red 4G 70, are
designed for use in solventborne, waterborne and industrial coatings. The coloristic properties of the quinacridone
‘pigment twins’ Hostaperm Red Violet ERX in solventborne coatings and Hostaperm ERX-WD in waterborne
coatings correspond closely to each other and facilitate the transfer of shades from one system to the other.
Clariant’s Colanyl range of VOC-free and binder-free pigment pastes offer improved environmental compatibility.
Increasingly stringent environmental regulations are promoting the replacement of heavy metals such as lead,
chromium and cadmium in pigments. Commercial alternatives such as bismuth vanadate and some diketo pyrrolo
pyrrole (DPP) pigments now offer colors that were once characteristic of the heavy metal pigments. BASF’s
(Mount Olive, NJ) Paliotan Yellow pigments are co-finished pigments based on bismuth vanadate and a high-grade
organic pigment, and economically combine the advantages of inorganic pigments (high hiding power) and organic
pigments (chroma and tinting strength).
BASF’s Xfast stir-in pigments are intended for waterborne architectural and industrial coatings. The stir-in pigments do not require the time-consuming dispersion steps needed to incorporate conventional powder pigments into coatings. The granular pigments generate very little dust during processing and disperse evenly and quickly into waterborne systems.
In Constellation Colors, BASF introduced the use of retroreflective pigments for automotive coatings that illuminate
the coated substrate when exposed to a light source, such as headlights from an oncoming vehicle. Another
approach in this concept was the use of glow-in-the-dark electroluminescent pigments that are controlled by a
low-voltage electrical charge.

Report Abusive Comment