There are several synthesis routes known to produce such nanoparticles, but they often result in either low yields or in materials that need further processing to show optimum performance. This can lead to high material prices, and in combination with increasing raw material costs, the overall costs can become prohibitive for some applications. An example of this would be the use of silver. The average price of silver has been steadily increasing during the last few years, and silver nanoparticles are even more expensive. For coatings applications, most companies now focus on non-metallic compounds, like carbon materials, to achieve electrical conductivity.
Carbon exists as different allotropes, e.g., diamond, graphite, fullerenes and carbon nanotubes. While diamond consists of sp3 hybridized carbon with a cubic crystal lattice, all other known allotropic forms contain sp2 hybridized carbon atoms, and thus they are preferred for electrical conductivity. Since their observation in 1991 by Iijima, carbon nanotubes have been the focus of considerable research.1 Scientists have since reported remarkable physical and mechanical properties for this fascinating allotrope of carbon.2
|
| Table 1 Click to enlarge |
From unique electronic properties to mechanical properties that exceed any current material, carbon nanotubes offer tremendous opportunities for the development of new material systems. In particular, the excellent electrical conductivity of carbon nanotubes combined with their high aspect ratio offer potential for the development of functional coatings. This article reports on recent advances in using additives based on carbon nanotubes to enhance the electrical conductivity of several coating systems.
Experimental

|
| Table 2 Click to enlarge |
All coatings were applied on glass panels or PET foils with wire bars at 75 µm wet film thickness. After a curing procedure, all panels were stored for 24 h in an acclimatized chamber, and surface resistivity was measured three times at each panel.
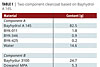
Starting formulations are shown in Tables 1-3. Multiwall carbon nanotubes from different suppliers were used in the form of aqueous dispersions.
Results and Discussion

|
| Table 3 Click to enlarge |
To determine the performance of multiwall carbon nanotubes (MWCNT) in different aqueous coating systems, we used a two-component clearcoat based on Bayhydrol A 145, and two one-component baking systems based on Worleesol 61 A or Bayhydrol D155, respectively. The A 145 system started curing immediately after application, while curing of the baking systems was initiated by elevated temperature. The two baking systems offer different resin chemistries; alkyd/melamine in Worleesol 61 A and polyester/melamine in Bayhydrol D155. The difference between the three systems had an influence on the distribution of MWCNT inside the coating, resulting in different percolation thresholds.
In all coatings, dosages of 0.5% to 8.0% MWCNT (calculated on solid resin) were incorporated, and films were applied on glass and PET substrates using wire bars to achieve 75 µm dry film thickness. Films with 0.5% to 2% MWCNT were already quite dark but still translucent, while higher MWCNT concentrations resulted in nontransparent coatings (Figure 1).

|
| Figure 1 Click to enlarge |
As seen in Figure 2, the lowest percolation thresholds were observed in the D155 system, followed by the Worleesol 61 A system. Even at low MWCNT concentrations of 0.5% to 2%, anti-static properties were achieved, and concentrations of 2% to 4% MWCNT resulted in electro-conductive coatings with surface resistivity below 105 W.
A possible explanation for this finding follows. The MWCNT were incorporated into the coatings in the form of dispersions in which the carbon nanotubes were homogeneously dispersed and stabilized by wetting and dispersing additives. This perfect distribution was transferred into the coating system. In the two-component system, curing started directly after application, which kept the carbon nanotubes in a very well-dispersed state. It resulted in a higher percolation threshold since many MWCNT were in an isolated position and were not contributing to the percolation network. The baking systems started curing after the flash off time and while applying heat. This enabled the MWCNT to agglomerate on a very small scale to achieve percolation. It can also be described as a kind of self-organization, and it seems that the resin chemistry also contributed to this process. Unfortunately, we could not yet clearly see differences in distribution by REM microscopy to prove this theory.

|
| Figure 2 Click to enlarge |
In addition to the influence from the resin chemistry or curing process, there was a significant contribution due to the particle size, aspect ratio and surface chemistry of the conductive pigment itself. We used two different kinds of multiwall carbon nanotubes. The first one, MWCNT 1, exhibited some structural defects and some oxygen-containing groups on the surface. The second one, MWCNT 2, had less structural defects and a less polar surface, as well as a slightly longer particle length. As a comparison, we used a conductive carbon black, which offered a polar surface and particle agglomerates with low aspect ratios compared to MWCNT. Figure 3 shows that MWCNT 2 offered lower percolation thresholds compared to MWCNT 1 in the D155 system.
To achieve the same level of surface resistivity, one would have to use between 8% to 10% carbon black, 2.5% MWCNT 1 or 1.5% MWCNT 2. This effect becomes even more predominant in the Bayhydrol A 145 system where MWCNT 1 gave almost no surface resistivity improvement. Due to their more polar particle surface, the carbon nanotubes kept their well-dispersed status inside the two-component clearcoat system and even at high concentrations of 8%, there was almost no improvement in conductivity. The MWCNT 2 had a higher aspect ratio and resulted in a percolating network, exhibiting surface resistivity of 106 Wat a MWCNT 2 concentration of 6%.

|
| Figure 3 Click to enlarge |
It is obvious that the performance of carbon nanotubes as well as any other conductive pigment strongly depends on several parameters. First, is the interaction between the conductive pigment and the coating matrix. If the affinity of the resin to the conductive pigment is too high and the conductive pigment stays in a perfectly dispersed status, the formation of a percolating network is hindered. Therefore, the conductive pigments should be used in the form of dispersions to guarantee a good initial dispersion status. In addition, the wetting and dispersing additives used have to guarantee good stabilization as well as suitable compatibility towards the coating matrix. Percolation will take place when the conductive pigments are allowed to agglomerate on a molecular state, still keeping a good distribution. Strong flocculation has to be avoided; otherwise the necessary amount of conductive pigments would be too high. There has to be a good balance between optimum distribution and minimum agglomeration.
|
|
| Figure 4 Click to enlarge |
The second point to be considered is to use conductive pigments with high aspect ratios to achieve percolation at low concentrations. Therefore we believe that carbon nanotubes can offer a strong benefit compared to carbon blacks due to their structural uniqueness (Figure 4).
Compared to organic anti-statics (for example, those based on quaternary ammonium salts), conductive pigments offer permanent anti-static or electro-conductive behavior to coatings. Quaternary ammonium salts can create a thin water film on the surface due to their high polarity, but their ions can migrate out of the coating matrix, often resulting in decreasing performance over time. Carbon nanotubes, especially, can overcome this drawback due to their high aspect ratio and their entanglement tendency. Figure 5 shows the surface of a clearcoat with 0.1% carbon nanotubes. The carbon nanotubes appear on some spots on the surface and disappear into the matrix again to form a three-dimensional network. This guarantees good surface conductivity, and the carbon nanotubes are strongly fixed into the matrix.

|
| Figure 5 Click to enlarge |
Conclusion
To increase the conductivity of coatings, carbon nanotubes can offer an interesting alternative to the classical conductive pigments like carbon black or metallic particles. To get the optimum benefit from this fascinating material they should be incorporated into the coating in the form of dispersions to guarantee optimum distribution. In addition, the right wetting and dispersing additives have to be used to enable percolation and compatibility with the coating matrix. Further investigations will be done to enable coatings manufacturers to predict the interaction between coating ingredients and the carbon nanotubes and the resulting performance.
References
1 Iijima, S. Nature354 (1991)56−58.
2 Collins, P.G.; Avouris, P. Scientific American 283 6 (2000) 62–69.





Report Abusive Comment