This paper was presented at the 39th Annual Waterborne Symposium in New Orleans, 2012.
A mixture of proteins, available as GRAS (generally regarded as safe) foodstuffs, has been identified for a variety of uses in UV-curable coatings. For example, the protein mixture may be used with a water carrier as a self-photoinitiating film former. Other GRAS additives may be used to resist oil or grease. The mixture may also be used as a pigment extender, which also aids in cure, or as a photoinitiator or co-initiator with more conventional UV-curable components. This article describes some of the early research using this new technology.
Types of UV Curing
There are three types of chemistry generally used in UV curing. By far the most common type is free radical curing, which is most often applied to acrylates and methacrylates. Free radicals of the R* form are generated by using a photoinitiator such as benzophenone. The free radicals attack an unsaturated bond, which may be opened so that affected molecules can be crosslinked.
A second type is cationic curing. With the addition of UV light and a photoinitiator, such as an iodonium or sulfonium metallic salt, a Bronsted acid is generated. Usually an epoxide ring is opened and linked to a polyol. In UV-curable coatings, free radical and cationic are the most commonly used types of curing.
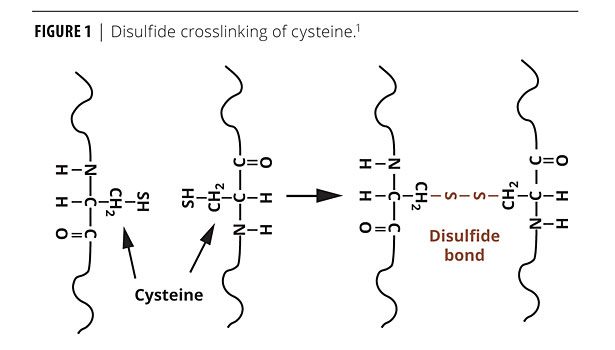
The third type of UV curing involves the use of thiol chemistry. This is the type of curing most relevant to this article. With the addition of UV light and optionally another photoinitiator, hydrogens are removed from S-H functionalities. At this point, a sulfur-containing charged species is formed. This species may attack a double bond, a process known as thiol-ene coupling. It may also bond with another sulfur, forming an S-S bond. Both of these processes are discussed in this article. When acting purely as a photoinitiator, we can expect a sulfur-containing charged species to act upon a double bond. When dealing with the formation of a self-photoinitiating film formed by proteins, S-S bonds will form. The S-H functionalities in proteins are found on the amino acid cysteine. Disulfide bonding of cysteine is shown in Figure 1.
The Germ of an Idea
Although first explored in the 1930s, food irradiation research grew in the 1950s under the “Atoms for Peace” program. The first petition for treatment of foods by irradiation was submitted in the 1960s.2 A task group established in 1981 concluded that studies with irradiated foods do not show adverse toxicological effects.3 Nonetheless, over the years there has been considerable concern over the effect of ionizing radiation on foodstuffs. A study in 2000 explored the structural changes, such as crosslinking induced in ovalbumin, ovomucoid and ovotransferrin by the effect of oxygen radicals generated by gamma radiation.4 It occurred to this researcher that if such changes could be induced by the use of ionizing radiation, perhaps similar changes could be induced with UV radiation.
There are two reasons to explore the use of UV radiation to create coatings from foodstuffs. One is increasing public demand for more nonpetroleum-based products. Bio-based content is of increasing interest. The second reason is the absence of UV-curable materials acceptable for direct food contact. There are a few acceptable under FCN 772, but even these require testing of extractables.5
FCN 772
The RadTech Food Contact Notification Alliance received FDA clearance on March 8, 2008, for several monomers, an oligomer and a photoinitiator. These components may be cured by either UV or electron beam (EB) radiation. Formulations using these components may include any other reactants or other substances permitted for the intended use under 21 CFR. These UV or EB formulations may be used as coatings, inks or adhesives. Substrates include polymers, paper and paperboard, and metals. Coatings, inks and adhesives so formulated are permitted a migration level of up to 1 ppm for each monomer or photoinitiator. However, the total level of nonvolatile extractables from a finished coating may not exceed 1 ppm after correction for migration levels of whatever monomer or photoinitiator was included in the formulation. The specified UV- and EB-cured coatings and inks may be used in direct contact with food, subject to these requirements.
FCN 772 does not apply to every user of these materials. Under FDA regulations, only members of the Alliance and their customers may claim clearance for materials and or formulations covered under FCN 772.6
Many businesses find these requirements difficult to meet. Coatings created from foodstuff materials that are generally regarded as safe under 21 CFR 175 would create no such difficulties.
Self-Photoinitiating Film Former
Proteins containing the amino acid cysteine contain S-H functionalities. Albumin is one such protein. While most are familiarly found in animal products, albumin and other proteins may be extracted from vegetable products such as oats. The S-H bonds from cysteine may be oxidized to form S-S bonds. Natural proteins are usually tightly curled. Such a structure may shield S-H bonds from reactions. A curled structure may be relaxed mechanically, thermally or by treatment with a GRAS mild acid such as 2,3-dihydroxysuccinic acid, ethanoic acid, 3-hydroxypentanedioic acid, salts of these acids or mixtures thereof. Albumin, transferrin, ovomucin, lysozyme or combinations of these proteins in powdered form may be mixed with a mild acid and dissolved in water.
Applications
Air Barrier
The mixture may be applied to a substrate such as paper. Curing may be accomplished by passing the substrate under a UV light having radiation from 200 nm to 400 nm. A common wavelength would be 280 nm. A peak absorption may be seen at 254 nm. The temperature should not exceed 70 °C so that coagulation does not take place. Such a coating will produce some resistance to the penetration of air. An early project involved decreasing the amount of air passing through a paper product to minimize the amount of combustion that could take place in a product placed inside the paper. Variations in formulations were tried. The amount of solids in the water carrier was adjusted from 17% to 23%. Formulations were tried with and without a GRAS emulsifier. Coating weights and curing speeds were varied as well. Diffusion constants through coated paper varied from 1.1 cc/min/cm2 to 0.3 cc/min/cm2 at 1 cbar pressure. It appeared that these numbers could be further improved. However, the project was abandoned due to issues unrelated to the coating. A related application that might be a topic for further exploration would be the slowing or prevention of the diffusion of odors and aromas in packaging.
Flavored Coatings
For a flavored coating, an oil such as peppermint oil may be adsorbed on a GRAS starch product and added to the mixture. A GRAS starch product may be used as a sole additive to increase barrier qualities to such substances as grease. Natural gums may also be used as additives, as well as GRAS dyes, emulsifying agents, vegetable fillers and de-foamers.
Grease Resistance
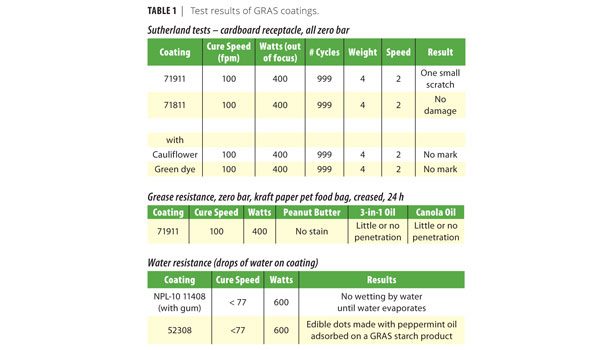
Special attention was paid to the development of grease resistance. At present, bags for greasy foods such as dry pet food use barriers such as polyethylene to prevent the migration of grease to the outside of the bag. Using the protein mix as the basic film former, various additives were used to block such migration. Since the creasing of bags may crack a coating and allow grease to pass, all testing was done on creased paper. A kraft paper, such as that used in pet food bags, was used for test purposes. Peanut butter,3-in-1® oil, and canola oil were used as test reagents. These three substances were allowed to sit directly on a crease in the coated paper for 24 h. Absorbent paper was placed beneath the test samples to detect penetration. Samples of these same coatings were additionally tested for abrasion resistance using a Sutherland rub tester with a corrugated cardboard receptacle.
Fillers
Various powdered and dehydrated foods were employed as fillers for coatings using the protein-forming film. Cruciform vegetables were of particular interest due to both availability and cellulose content. Fillers seemed to contribute to abrasion resistance. These coatings were also tested on a Sutherland rub tester in the same manner as the grease-resistant coatings.
Edible Inks
Edible dyes were also employed to produce colored coatings that might function as inks. The colors used were dispersed in propylene glycol using lecithin as an emulsifier. The protein mix was combined with a gentle acid component. Water was used as a carrier. These coatings were applied to ink jet stationary for testing. The coatings were quite resistant to abrasion, but soluble in water. The coatings were also edible, and some were sampled by this researcher.
Water-Resistant Coatings
Water-resistant coatings were developed using the protein mix, a mild acid and natural gums. Natural gums such as guar and xanthan gum are often used as thickeners (Figures 2 and 3). This process may be accomplished because many gums have OH functionalities that enable them to form hydro-colloids. Water is held at the surface, allowing foodstuffs such as sauces and gravies to thicken. Although usage of gums in this way has historically been more popular in commercial production than with the home cook, the recent popularity of “molecular gastronomy” has seen gums making their way into many home and restaurant kitchens. Thus they are now fairly common items. Another important usage of gums, particularly xanthan gum, has been in the addition to baked goods that do not contain gluten. Yeast-raised baked goods without gluten are very difficult to make without these additions because they are missing the long elastic strings of protein that trap the carbon dioxide produced by fermentation. Gums aid in the development of a suitable gas barrier so that the bread may rise. When used in protein film-forming coatings the gums may act in at least two ways. One is to loosely bond to water to spread it out at the surface of the coating instead of passing through it. The other is to provide actual barrier qualities.
Edible Dots
Edible dots are a very interesting application. Any edible oil such as peppermint may be used. The oil is adsorbed on a GRAS starch product, forming a powder. The powder is then mixed with the protein mixture and a mild acid. Water is used as a carrier. The amount of water may be very low, yielding a paste. The dots may be applied to the interior of packaging to add extra flavor, aroma or other properties. Many herbs have been shown to have inhibitory effects on microorganisms. These include everything from allspice and cinnamon to rosemary and sage.9 Oils from such herbs may likewise be adsorbed and incorporated into coatings. The efficacy of such coatings for the inhibition of microbial growth remains to be tested.
A sampling of coatings prepared in these various manners produced the results shown in Table 1.
Pigment Extender
The use of pigments in UV-curable coatings presents a unique challenge. Pigments can absorb the very UV light that is meant to cure the coating. The UV industry gets around this problem with the use of two approaches, which may be employed separately or in combination. The first approach is the use of doped lamps. Elements such as iron or gallium are added to mercury vapor UV lamps. The addition shifts the spectral output in such a way that frequencies are produced that are not absorbed by whatever pigments are being used. Generally, iron-doped lamps are used for dark pigments such as carbon black, and gallium-doped lamps are used for light colored pigments such as titanium dioxide.
The second approach involves the use of photoinitiators that are activated by frequencies not absorbed by the pigments. For example, 2,4,6-trimethylbenzoyldiphenyl phosphine oxide(TPO) may be used to cure white pigments. Isopropylthixanthone (ITX) is used for dark pigments. A combination of approaches would combine TPO with a gallium-doped lamp or ITX with an iron-doped lamp.
Unfortunately a number of facilities, especially in the printing industry, do not use doped lamps. In addition, photoinitiators designed to cure pigmented coatings are often more expensive. In the case of ITX, there have also been fears raised of migration into food products, even without direct food contact.
The use of protein mixtures as pigment extenders presents a different approach. Cure is actually promoted by the mechanism of thiol curing instead of being impeded. With the addition of the protein mixture, it is possible to use less pigment and less expensive photoinitiators. Doped lamps may not be required.
We can look at cases on two different substrates. The first case is an application to metal by roll coat. Cure speeds were upwards of 100 feet per minute, which is very fast for a UV metal coating. Traditional acrylates were used as well as a pigment containing titanium dioxide bonded to a solid acrylate monomer. The protein mix was also added. The protein mix allowed the coating to cure at higher speeds with better coverage than the titanium dioxide pigment by itself. The use of gallium lamps was eliminated or reduced, depending on cure speed.
The second case involves the application of a textured hard coat to a corona-treated PET surface. The coating was applied by gravure and cured at speeds up to 225 feet per minute. A standard mercury UV lamp was used. Application thickness ranged from 7 to 19 bcm or about 0.15 mils. The primary goal of the project was to produce a rough textured coating with scratch resistance to 0000 steel wool. The protein mix was combined with the aforementioned titanium dioxide pigment in a 2:1 ratio. A coating was prepared with a multifunctional urethane acrylate and a monomer mix with a variety of functionalities. Additional silica fillers were used as well. The resulting coating showed a rough texture with an unusual appearance. The surface could not be scratched with 0000 steel wool.
Matting Agent
The protein mix may be used as a slightly rough textured matting agent. In one case, the presented problem was of matte rubbing off an aluminized label surface. The mix of proteins was added, along with a slightly acidic adhesion promoter, to a traditionally formulated UV topcoat for labels. This produced a label that could stand up to an excess of 1000 Sutherland rubs without losing matte on the aluminized surface.
Photoinitiator or Co-initiator
As previously mentioned, some UV-curable acrylates have been approved under FCN 772. They are tripropylene glycol diacrylate (TPGDA), trimethylolpropane triacrylate (TMPTA), trimethylolpropane ethoxylate (TMPTA) and epoxy diacrylate. In addition, one photoinitiator, Esacure One, has also been approved. These components may be combined with any reactants previously approved for food contact. Also, as previously mentioned, limits on extractables apply.10
The use of a GRAS photoinitiator will increase the number of possible formulations and decrease the number of extractable measurements necessary.
Tests were performed with TMPTA and TMPEOTA. Both TMPTA and TMPEOTA could be cured, although slowly, with the protein mix.
It is even more interesting to note that the amino acid cysteine, even when not acting as a component of the proteins, may be used as a curing agent when mixed with TMPTA or TMPEOTA.
Cysteine is dispersed rather than dissolved in these monomers, producing a milky appearance. The monomer readily impregnates the paper. Curing is much more readily accomplished with a very thin coating. The coatings have a smooth, almost slick feeling. This is in direct contrast to the free radical curing normally used with acrylates in which curing of very thin films is inhibited by the presence of oxygen.
Curing with cysteine alone is done somewhat differently than with cysteine incorporated in a protein. The unprotected cysteine is very sensitive to oxidizing agents, including acids. Therefore, acids are not added as a matter of course. Furthermore, the amount of acidic material in a formula must be minimized to prevent gelling.
Conclusion
The use of GRAS materials can provide an interesting addition to the formulating toolbox for UV-curable coatings. Many aspects of the use of these materials remain to be explored. Among them is further exploration of the diffusion of air and odor through self-initiating protein films. The addition of anti-microbial herbal oils to the aforementioned films is also an area to be investigated. This fledgling technology may provide many opportunities for research and the development of novel UV-curable coatings.
References
1 UCI Biology Lectures, http://bass.bio.uci.edu/~hudel/bs99a/lecture26/lecture7_3.html, (accessed 11/15/2011).
2 Pauli, G.H.; Takeguchi, C.A. Irradiation of Foods – An FDA Perspective: Food Reviews International, Marcel Dekker, Inc., 1986, 88.
3 Pauli, G.H.; Takeguchi, C.A. Irradiation of Foods – An FDA Perspective: Food Reviews International, Marcel Dekker, Inc., 1986, 96.
4 Moon, S.; Song, K.B. Effect of Irradiation on the Molecular Properties of Egg White Proteins, Food Sci. Biotechnol., 2000 Vol 9 No 4, 239-242.
5 Radtech Resources Page, http://radtech.org/whats_new/FCN.html (accessed 7/23/2009).
6 Radtech Resources Page, http://radtech.org/whats_new/FCN.html (accessed 7/23/2009).
7 Scientific Psychic Page, http://www.scientificpsychic.com/fitness/xantahn.gif (accessed 10/26/2007).
8 Rajpurohit, H.; Sharma, P.; Sharma, S.; Bhandari, A. Polymers for Colon Targeted Drug Delivery, Indian Journal of Pharmaceutical Sciences, 2010, volume 72, Issue 6, 2.
9 Snyder, O.P. Hospitality Institute of Technology and Management, http://www.hi-tm.com/Documents/Spices.html, (accessed 10/29/2007).
10 Radtech Resources Page, http://radtech.org/whats_new/FCN.html (accessed 7/23/2009).
For more information, contact Sally Ramsey at sally.ramsey@ecologycoatings.com.



Report Abusive Comment