Fumed silica is an example of a nano-scale inorganic additive that can be particularly enabling in polymer film and coating systems, especially in WB formulations. The fractal-like shape and high specific surface area of fumed silica provide viscosity control, which is a function where fumed silica is more commonly employed.1 However, this feature of the fumed silica structure, when properly dispersed within the WB coating formulation, can aid in polymer latex film formation. Such particle additives can also help increase drying rates to improve processing throughput times. They can also help strengthen the film and relieve stress by altering the local micro-rheology and drying dynamics2 to mitigate crack formation during the latex polymer coagulation and coalescence process that occurs during the drying of the coating.3,4 Furthermore, unlike volatile organic additives commonly employed as leveling agents, plasticizers and co-solvents that evaporate upon drying, particles like fumed silica remain in the coating and can fulfill additional functionalities such as mechanical reinforcement, while helping make the overall formulation more “green.”
As produced and supplied commercially, fumed silica can be difficult to work with because it exists mostly as powdery agglomerates with high specific surface area that are not easily separated or dispersed into individual particles. We have developed a proven method for dispersing fumed silica or fumed alumina into individual particles in water for efficient utilization in polymer latex systems. The product line is produced under the trademark CAB-O-SPERSE®, and is available in a range of concentrations, particle sizes, particle surface charges and different pH ranges for use in a variety of applications.
Due to the superior degree of dispersion down to the nano-scale, CAB-O-SPERSE products lend themselves to coating applications. Dispersions of fumed silica facilitate addition to coating formulations, and they can yield superior mechanical performance relative to their dry powder counterparts. Such additives provide microscopic polymer entanglement points in the coatings that limit polymer chain mobility, which can help improve coating mechanical properties such as tensile strength, block resistance, hardness, and scratch or abrasion resistance.5
Polymer Latex Reinforcement
Nanoparticles are well known to provide enhanced mechanical properties in polymer films and coatings.5,6 The final state of dispersion and distribution within the polymer film has a significant impact on the ability of the particle additives to enhance performance of a coating system. Superior states of dispersion provide the greatest degree of interparticle separation within the continuous phase, and a most effective use by mass of the additive to provide enhanced mechanical properties in the final coating product.6
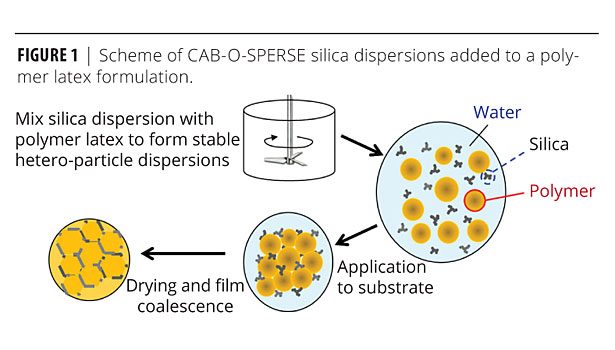
During the dispersion process, fumed silica aggregate particles are stably broken down to small sizes of 50-300 nm (as measured by light-scattering techniques) that are extremely difficult to destabilize. Adding a silica dispersion like CAB-O-SPERSE to a polymer latex formulation results in very stable mixed particle dispersions that maintain superior distribution of the inorganic additives in the host polymer, even upon drying (Figure 1). The silica particles remain well distributed and, depending on the relative time-temperature profile during drying, can remain at the vestigial latex interparticle boundaries post-coalescence or can inter-diffuse throughout the polymer matrix.7
The basic physics of the effect due to silica in the polymer emulsion or latex remains largely true regardless of the polymer latex used or the end-use application. Cabot has previously studied this latex reinforcement phenomena in various natural and synthetic rubber emulsion systems and demonstrated superior mechanical properties in dry products relative to the base rubber when predispersed silica particles are added to the latex rubber formulation.7 Recently, to support the generality of this fumed silica nanoparticle reinforcement concept, Cabot studied a model system of a high-glass-transition-temperature (Tg) non-coalescing polystyrene (PS) latex. This was used to demonstrate the interaction of CAB-O-SPERSE with a polymer latex system during formulation and after hetero-coagulation and drying. The high-Tg PS latex was chosen intentionally to allow better scanning electron microscopy resolution of individual particles since the latex did not coalesce into a film. After adding the CAB-O-SPERSE to the latex using gentle agitation, the mixed system remained very stable with no evidence of settling.
In the model PS system, upon hetero-coagulation the silica particles retain their distribution and there is little evidence of phase segregating into silica-rich domains within the polymer (Figure 2). Figure 2 contains electron micrographs showing evidence of the good distribution of silica in the host polymer, even at high loadings. The polystyrene latex particles are the round, spherical particles approximately 50-100 nm in size. The fumed silica particles are the slightly brighter, fractal-string-like smaller particles dispersed throughout.
This preserved state of additive dispersion after film formation has important ramifications when it comes to balancing trade-offs in terms of nanoparticle loading versus desired physical properties in the coatings. The superior degree of dispersion that CAB-O-SPERSE can provide may allow more or less silica to be used to match or exceed various performance needs and break trade-offs between functional coating requirements. This evidence confirms what Anand, et al.7 reported in their silica reinforcement studies, and suggests that this phenomena should hold true in a wide variety of polymer latex applications including WB coatings and adhesives.
Performance Studies
Our applications labs have been working with proxy WB industrial coating formulations containing fumed silica as a reinforcing additive based off of Neocryl A 6085 aqueous latex acrylic resin. The formulation is listed in Table 1. The main purpose herein was to observe performance results in more relevant coating systems. Comparisons and studies were made of coatings from CAB-O-SIL M-5 fumed silica powder-based sample formulations and of coatings prepared from using a grade of CAB-O-SPERSE fumed silica dispersion, 2017A, which contains an equivalent fumed silica particle provided in a pre-dispersed form. The powder was ground in an aqueous mill-base using a high-shear mixer in our applications lab to an equivalent solids of commercial product 2017A, 17% wt. in water. Then, either the lab-made mill-base or the commercial 2017A dispersion was added to the latex coating formulation to achieve a desired net silica loading.
All coatings were deposited on substrates using a 3 mil draw down bar to yield coatings of approximately 1.0-1.5 mil dry thickness. Coatings were deposited onto 2x3-inch glass substrates or onto B1000 steel coupons. The coated glass substrates were used for transparency and clarity tests as well as for nano-indentation to quantify mechanical properties of the coatings. The pretreated B1000 steel panels were used for other tests like corrosion resistance in salt-spray chambers, and other mechanical tests.
Optical Properties
Prior to formulation, just after preparation, the starting particle size distributions of the silica in the mill-base and in CAB-O-SPERSE 2017A respectively, were quite similar, as shown in Table 2. However, over time, CAB-O-SPERSE products remain extremely stable and do not change in size, whereas the mill-base of silica shows significant agglomeration and settling, which in turn can help explain the poor clarity and high haze in the coatings.
The importance of this finding is tied to the consistency of the CAB-O-SPERSE performance. The lack of variability and consistent performance is the result of decades of experience developing the CAB-O-SPERSE product family to its current state. While the immediate mill-base may look and act like CAB-O-SPERSE, and it may be suitable for certain applications, it will not have the prolonged shelf-life, or the same reliable stability in formulation.
The mill-base’s stability alone and in the formulation was less than the 2017A dispersion, as is readily apparent in the particle size data in Table 2, and the percent haze data shown in Figure 3. Coatings were measured with a Hunter Lab UltraScan Pro under normal incidence of the light source. High haze values indicate a corresponding lack of clarity or transparency. The significance of Figure 3 is that it demonstrates that in the right formulation, CAB-O-SPERSE can be well-suited for WB clearcoats, and related applications where high clarity and gloss retention is important to the coating function. This is primarily attributed to the superior state of dispersion and the retention of that distribution as the silica nanoparticles distribute themselves in the host polymer coating.
Mechanical Performance
Nano-indentation is a method for quantifying the force to displace a probe or scribe head of a known size into a surface. This force per area of the probe required to indent or scratch a surface is effectively a measure of the hardness of the coating. Nano-indentation experiments were performed with a Nanovea Nano Module (P-Macro/Nano) following the guidelines proscribed for ASTM E-2546. The hardness of the coating increases as a function of the added silica (Table 3). Note that within error, as shown in Table 3, the hardness of the sample acrylic coatings effectively doubles between 1% wt. silica and 5% wt. silica added to the coating, and is in excess of three times harder when 10% wt. silica is incorporated into the coating.
The ability to alter the coating hardness can be important for block resistance, abrasion resistance, tack reduction and anti-dirt pick up, all of which are critical for coating durability and consistent performance in applications. By using CAB-O-SPERSE rather than the dry powder, greater reinforcement and hardness can be obtained while achieving the same clarity (Table 3 and Figure 3). Mechanical and optical properties can be tailored by using CAB-O-SPERSE to balance trade-offs in performance requirements.
Corrosion Resistance
Corrosion protection and resistance is an especially important function for industrial metal and automotive coatings. To test for such performance, the coated B1000 metal panels were subjected to a neutral salt spray accelerated exposure chamber after they were scratched with a scribe per ASTM D1654. These coated panels were checked periodically while in the chamber for evidence of corrosion, and how the corrosion of the substrate under the coating propagated over time.
Figure 4 contains images of the scratched coatings and how they performed under this corrosive environment. Although the fumed silica in CAB-O-SPERSE is hydrophilic, and the coating formulations are all WB, there is still corrosion protection provided by these coatings. Furthermore, the corrosion resistance is equal to, if not greater than, the control (possessing no silica) up to about 5% wt. silica in the coating. Beyond that loading level, the corrosion prevention is poor and the presence of silica appears to become detrimental to corrosion protection. We attribute this performance to the reinforcing ability of the silica, which causes polymer entanglements that stiffen and strengthen the polymer, yielding coatings with better barrier properties that reduce the ability of water to diffuse through the coating and create corrosion pathways. Hence, contrary to popular belief, by using the proper loading for a given system, hydrophilic fumed silica particles can aid with corrosion resistance.
Conclusions
CAB-O-SPERSE is an effective WB coatings additive that can help maximize utility of fumed silica in application performance by providing superior nano-scale dispersion that yields uniform distribution of the silica within the final polymer coating. Optimizing the silica distribution in the host polymer yields enhanced properties that can potentially help WB coatings bridge some of the gaps toward SB coatings. CAB-O-SPERSE products are safe to handle, easy to use, have low variability and high consistency. These characteristics enable them to play a role in many properties throughout the product lifecycle from liquid formulation (like viscosity control), through application and coating deposition (like shear thinning behavior and stress relief in drying), to end-use functionality (like improved optical or mechanical properties). Additionally, the results show that CAB-O-SPERSE can help balance trade-offs in performance, depending on the functional needs the coating must provide in the end-use application.
References
1 Heilein, W. et al., Additives for Waterborne Coatings, Vincentz Network, Hannover, Germany, 2010.
2 Roberts, C.C.; Francis, L.F. Drying and cracking of soft latex coatings, J. Coat. Technol.Res. online web release, 2012.
3 Keddie, J. Film Formation of Latex. Mater. Sci. Eng.R: Rep. 1997, 21 101–170.
4 Keddie, J.; Routh, A.F. Fundamentals of Latex Film Formation: Processes and Principles. Springer, NY, 2010.
5 Overbeek J. Polymer heterogeneity in waterborne coatings, J. Coat. Technol.Res. 2010, 7 (1) 1–21.
6 Akcora, P.; Kumar, S.; Moll, J.; Lewis, S.; Schadler, L.S.; Li, Y.; Benicewicz, B. C., Sandy, A., Narayanan, S., Ilavsky, J., Pappannan T., Colby, R. H., Douglas, J. F. “Gel-like Mechanical Reinforcement in Polymer Nanocomposite Melts”, Macromolecules 2010, 43, 1003–1010.
7 Anand, J.; Morris, M. Reinforcement of latex with fumed silicas” Rubber and Plastics News1997, March 10 issue.










Report Abusive Comment