Pushing the Edge of the Durable Color Envelope
New High-Performance Pigments for Lead Chromate Replacement in the Yellow and Orange Color Space





The trade-off in the yellow and orange color space between the weatherability, heat stability and opacity of inorganic pigments versus the chromaticity, brightness and tint strength of organic colorants, in light of the decrease in use of pigments based on deprecated metals, has opened an opportunity for new colored pigment chemistries. Two pigment chemistries can be useful in bridging the gap between the two groups. One of the chemistries is the recent commercialization and improvement of CI Pigment Yellow 216 and Orange 82, more conversationally called rutile tin zinc (RTZ) pigments. The other is a new class of yellow pigments called niobium tin pyrochlore (NTP), assigned the designation CI Pigment Yellow 227. Together these pigments provide high chroma, opacity and durability in the yellow-orange color space. These highly engineered pigments are excellent colorants for demanding thin-film applications and are compatible with a wide range of resins.
RTZ pigments were first developed in the 1980s. Improvements have come in the product class by increasing the redness to change the color from that of a red-shade yellow to more of an orange hue. Recent modifications have enabled the achievement of a true orange shade. Shepherd Color has developed a new product that expands the colors available with this chemistry. Orange 10C341 is the reddest version of RTZ in the marketplace.
NTP pigments are a brand new, unique, innovative and patented pigment class in the yellow color space. They have outstanding coloristic properties with a high-chroma red-shade, yellow masstone and high opacity. Their inherent inertness gives them excellent weathering and high-heat performance. They open up new color space that is not currently achievable in high-performance systems that do not contain toxic and carcinogenic materials. The first product of this new NTP class is called Yellow 10C151.
Both RTZ and NTP pigments make excellent coloring products by themselves or can be mixed with organic pigments in place of TiO2 or other inorganic pigments to make bright, stable, lead-, cadmium- and chromium-free opaque colors.
CICPs
Complex inorganic colored pigments (CICPs) are a specialized sub-section of pigments. They are commonly made from simple oxides that are then heated in a kiln around 600 °C and higher in either ambient or controlled atmospheres. At these elevated temperatures the metal ions transfer back and forth so that they are no longer simple oxides, but a matrix at the atomic level of two or more metal oxides. In this new chemical form they have new properties and are stable to their firing temperature.
An example would be the intimate mixing of a black cobalt oxide with white aluminum oxide and then putting the mixture into a kiln at about 1200 °C for a few hours. When cooled to room temperature they will chemically no longer be cobalt oxide and aluminum oxide, but rather a new chemical compound, cobalt aluminate (CI Pigment Blue 28) – a bright, red-shade blue with outstanding stability. The chemical make-up of CICPs is the major driver of the color of the pigment. The crystal structure, the elements in that crystal structure, along with trace metals all affect the wavelengths of light absorbed.1 The absorbed photons are converted into heat, and there are no double bonds to break, as in organic pigments.
While CICP-type pigments are among the oldest pigments to have been used by humanity, there still are important innovations and new applications for these very versatile pigments.
Rutile Tin Zinc (PY216)
The RTZ pigments run the color gamut from mid yellows to orange in tone. The most interesting of these pigments have bright, clean, red-shade masstones in the orange color space.
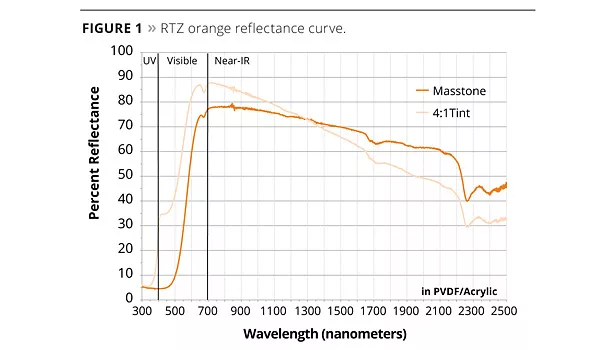
The closest standard CICP yellow to RTZ pigments are chrome titanates (PBr24), which are the workhorse red-shade yellow pigments for demanding applications. RTZ pigments have a more chromatic masstone and tint than standard PBr24 colorants. This chromaticity of RTZ Orange can be seen in the reflectance curves, with the steep rise of the curves giving the pigment a very high chromaticity and excellent red value (Figure 1).
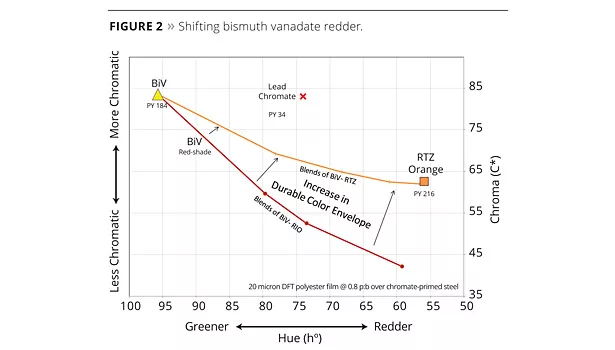
The RTZ pigment’s increased chromaticity makes it an excellent replacement for color matches containing PBr24 and organics, and allows for an increase in the color space that is achievable with standard colored titanates. RTZ orange also makes an excellent pigment to blend with organic pigments to improve opacity and weathering. Another interesting use for RTZ orange is in blends with bismuth vanadate (PY184) to shift them from green-shade to redder-shade yellows. Red iron oxide (Red 101) can be used to affect the same color shift but, as can be seen in Figure 2, the RTZ orange better preserves the chromaticity of these blends. Starting on the left-hand side of the plot, the line labeled “BiV-RTZ Blends” demonstrates how this approach produces more chromatic colors than the “BiV-RIO” line. Organic pigments could also be used to affect this color shift in PY184 pigments, but using an RTZ pigment keeps all the components inorganic with better opacity and durability potential. The RTZ orange pushes the edge of the durable color envelope.
The possibility of matching colors previously not attainable with standard inorganic pigments is bolstered by the inherent stability of the RTZ pigments. They have excellent resistance to acids and bases, are non-bleeding and are stable to around 320 °C. These excellent resistance properties give the RTZ orange good weathering results when tested in demanding applications in high-performance coatings (Table 1). PVDF-based coatings, because of their UV transparency, submit pigments to a high UV energy load. As can be seen in the table, after 3000 h exposure in a QUV cabinet the new RTZ Orange 10C341 performs similar to the long-time workhorse, but less chromatic, Shepherd Color Yellow 29 (PBr24). Due to its highly engineered properties, Orange 10C341 has excellent UV resistance and dissipates absorbed UV energy as vibrational energy and not with free-radical generation.
Niobium Tin Pyrochlore (PY227)
Lead chromate (PY34) use in industry is decreasing2 due to its inclusion on regulatory lists such as the European Chemicals Agency (ECHA) substance of very high concern (SVHC) list and also through market pressure. Matching the coloristic properties of PY34 has meant balancing properties of cost and performance such as durability, chemical stability and opacity. NTP pigments have been developed to fill the color space currently occupied by PY34. NTP yellow is newly patented and has recently gained regulatory approval for commercial sale. A CI Index number of PY227 has been assigned to the chemistry.
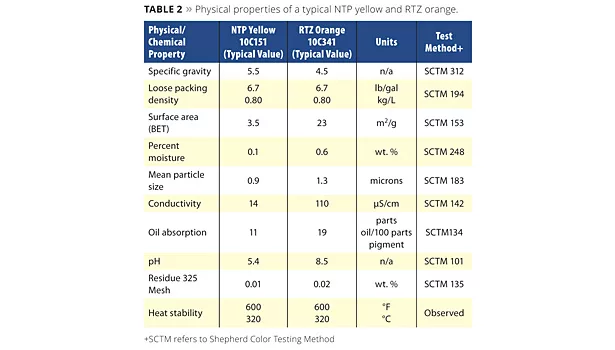
The most relevant intellectual property is U.S. Patent 8,192,541, including other patents, invented by Dr. Simon Boocock of the Shepherd Color Company.3 These pigments have the general formula of: Sn2Nb2O7. The physical properties of a typical NTP yellow and RTZ orange are shown in Table 2.
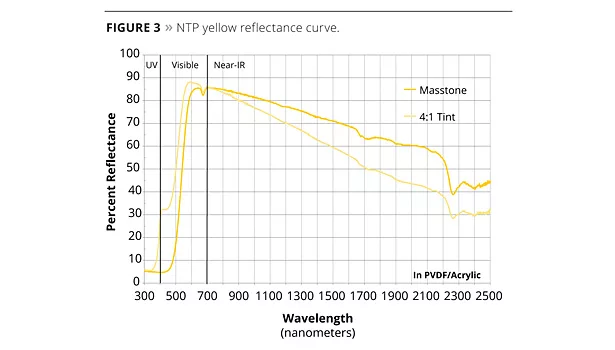
NTP yellow exhibits excellent temperature stability, low resin demand and surface area, fine particle size, ease of dispersion and generally desirable pigmentary properties. The key to its color can be seen in a graph of its reflectance curve (Figure 3).
The steep rise of the masstone curve from 460-580 nm is indicative of its near band-gap mechanism of producing color. The high reflectance past 600 nm continues on into the NIR, giving the NTP yellow an excellent total solar reflectance (TSR) of 73% for use in ‘cool’ materials that resist the solar heat build-up from the sun. The excellent selective absorption properties of the pigment, and its high coloring strength, are seen in the 4:1 tint curve, which shows a saturated yellow tint color.
The NTP yellow is a bright red-shade yellow similar in shade to middle chrome versions of PY34. NTP yellow is much more chromatic than standard titanates like nickel titanate (PY53) and chromium titanate (PBr24). It falls between bismuth vanadate (PY184) and orange versions RTZ (PY216) and is close to the redder shades of benzimidazolone (PY154). While falling in color space between these other pigments, it is not a replacement for those products because of its generally higher performance and being based on niobium. Plotting its place in color space with C* and h* values it can be seen that it falls in an advantageous color position (Figure 4).
The NTP yellow makes an excellent pigment based on its bright masstone, but it also makes an excellent colorant to blend with other inorganic pigments to make colors that before now had to be made with organic-inorganic blends or deprecated pigments. Just as the RTZ orange is blended with bismuth vanadate, the NTP-BiV blends offer an excellent way to make all inorganic pigment greener-shade yellow colors. These blends can be seen in the plot and are labeled ”Blends of BiV-NTP”. The NTP yellow can also be blended with RTZ orange to make outstanding all inorganic, highly durable red-shade yellow colors. These blends are represented by the NTP-RTZ line (Figure 5).
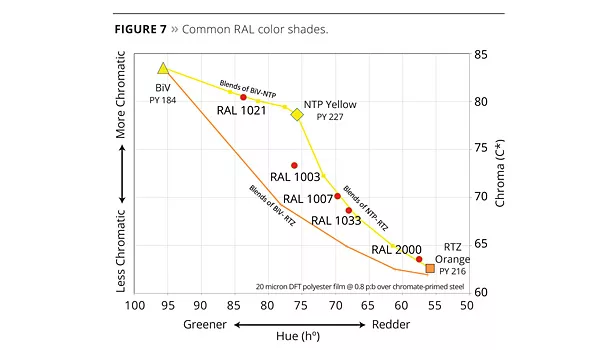
If we connect the BiV-NTP and the NTP-RTZ color blends lines at the NTP yellow color point, it forms a new high-chroma frontier in the highly durable color envelope when compared to the BiV-RTZ line. The area between the two groups of lines represents the increase in the durable color envelope since those colors, until now, needed to be matched by blending organic and inorganic pigments (Figure 6). In this color space are a number of very common RAL color shades (Figure 7).
One of those common colors is RAL 1003. There are a number of different ways to match the color that offer a different balance of properties, durability and cost. Before RTZ and NTP pigments, colors like RAL 1003 could be matched only with blends of organic and inorganic pigments. RAL 1003 can now be matched with a blend of the new NTP and RTZ pigments along with standard titanate pigments with increases in opacity and durability over other blends (Table 3).
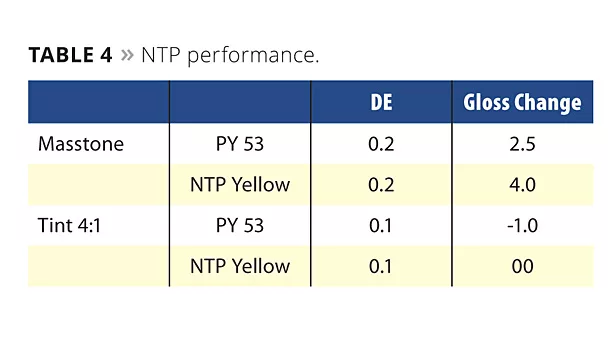
The NTP yellow pigment shows excellent resistance to acids and bases. When incorporated in a coating resin system and exposed in the periodic Kesternich acid stability test, the NTP yellow shows similar performance to workhorse pigments like PY53 (Table 4).
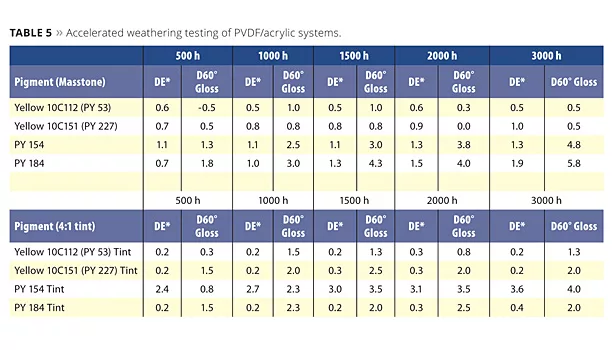
This stability is also shown in accelerated weathering testing. The PVDF/acrylic-based systems are very hard on pigments due to the high UV transparency of the resin system. As can be seen in Table 5, the NTP yellow has excellent color and gloss retention in accelerated weathering testing in a PVDF/acrylic system in both masstone and tint when compared to alternatives. The organic pigment PY154, which is close to the NTP Yellow 10C151 in chroma and hue, suffers degradation in a tint, while the inorganic pigments do well in the tint.
Regulatory Approvals
Currently the NTP yellows are TSCA approved for production and sale in the United States. Approvals for other registries are being obtained with eventual worldwide availability envisioned. Niobium compounds are not currently seen as a substance that gathers much concern beyond the normal safeguards needed for CICP pigments. See NTP Yellow MSDs for specific precautions and protective measures.
Summary
The RTZ orange and NTP yellow pigments provide interesting coloring options in the yellow to orange color space. While no pigment has exactly the same balance of properties as that of the lead- and chrome-based pigments, the RTZ and NTP pigments provide heat-stable, weatherable, chemical- and solvent-resistant alternatives that increase the durable color envelope for high-performance systems. They are especially well suited for powder, coil and extrusion coatings for use in ACE, high-temperature, automotive, high-profile branded color and other demanding applications. n
References
- Swiler, D. R. Scribd.com. Kirk-Othmer Encyclopedia of Chemical Technology- Inorganic Pigments, Copyright John Wiley & Sons, Inc.
- Charvat, R. A. Coloring of Plastics Fundamentals Second Edition. Hoboken, New Jersey: John Wiley & Sons, Inc, 2004. Print. Pg 135-136.
- U.S. Patent 8,192,541. “Substituted Tin Niobium Pyrochlore and Tin Niobium Oxide Pigments”. Simon Boocock, Ph.D.; 2012.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






