Paint producers have a number of bases at their disposal for adjusting pH. The most commonly used bases are amines, ammonia and/or caustic. Alkanolamine bases are most preferred, owing to their ideal neutralizing properties and generally low odor. Amine bases allow for the formation of colloid-compatible ammonium salts, while caustic leads to colloid-destabilizing sodium/potassium salts, as given by the following two chemical equations.

Caustic has the economic advantage of lower cost, but the incompatibility of caustic with many stable colloids makes its use problematic. The subject of alkanolamines versus caustic in paint has been discussed in a number of previous articles,1 wherein the use of caustic has been shown to result in increased water spotting and water sensitivity, decreased viscosity stability and general colloid instability. Many of these deleterious effects are related to an underlying mechanism of decreased colloid stability.
Emulsion Stability
The cosmetics chemist Weiner,2 among many others, has pointed out that emulsion stability depends upon the emulsifier collecting at the oil/water interface. Thus, a neutralized emulsifier can’t be too hydrophilic or too hydrophobic. The use of too hydrophilic a base (e.g., caustic) in the neutralization of, for example, polyacrylate emulsifiers, causes the ionic polyacrylates to become too soluble in the water phase. Amines, especially amines with balanced hydrophilic/hydrophobic properties, are better able to deprotonate the polyacrylate while still not making it too water-soluble.
An emulsion is created by rapidly mixing two immiscible liquids, e.g., oil and water. One liquid becomes a dispersed phase of small drops within a continuous phase of the other liquid. The difference in energy of a biphasic emulsion before and after mixing can be calculated as follows:
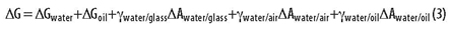

One can imagine an initial drop of oil suspended in the middle of a beaker full of water. The drop is rapidly mixed into the water to form an emulsion. The interaction of the two liquid phases (oil and water) with the air and the glass is approximately the same before and after. Looking at the five terms in the above equation, it is apparent that only the last term and the entropy (contained in Gwater and Goil) change significantly upon mixing. The energy of mixing can thus be approximated as follows:
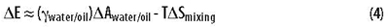
The DAwater/oil term represents the change in interfacial area between the two immiscible phases after mixing. The change in the interfacial area of contact between the oil and water is a consequence of creating the emulsion.
An idea of the magnitude of this interfacial area change can be obtained with a quick calculation, as shown in Figure 1. The surface area of a sphere is 4pr2, and the volume of a sphere is (4/3)pr3. If we imagine a drop of oil with radius of 1 cm (µ= micron, radius = 1x104 µ, surface area = 1.26x109 m2, volume = 4.19x1012 m3) being mixed into an aqueous phase until it is dispersed into drops of radius 1 micron (surface area = 1.26x109 m2, volume = 4.19x1012 m3, 1x1012 drops formed with a total surface area of 1.26x1013 m2), then the change in interfacial area is on the order of 10,000 fold. If this drop of oil is dispersed into 10 mL of water with an interfacial tension between the oil and water of 30 dynes/cm, then the total enthalpy of interfacial area change will be about 0.09 calories.
The entropy of mixing will usually be positive, and the term -TDSmixing will contribute to emulsion stability. The enthalpy change caused by the increase in interfacial area will also be positive, and the term gwater/oilDAwater/oil will result in emulsion instability, which promotes phase coalescence. The emulsion can become thermodynamically stable only when the interfacial tension gwater/oil is very low. Typical commercial emulsions (e.g., paint) are kinetically stable but thermodynamically unstable, and much of the time spent on improving the longevity of emulsions focuses on kinetic stabilization. Thermodynamically unstable emulsions are usable for the length of time it takes for the two phases to coalesce, while with further lowering the interfacial tension between the oil and water phases it may be possible to get a thermodynamically stable system.
Interfacial Tension
Liquid/gas and liquid/liquid interfacial tension measurements demonstrate that amines have a unique ability to reduce interfacial tension between immiscible phases. See, for instance, the room temperature aqueous/air surface tensions (determined by the maximum bubble pressure method) provided in Table 1. Calculated HLB values (% hydrophilic molecular weight (MW) based on total MW ÷ 5) for the amines are shown in the final row. The HLB is calculated as the ratio of the hydrophilic MW to the total MW multiplied by 20. This type of HLB calculation is commonly used for surfactants and can also be applied to lower-MW molecules.
The lower surface tensions exhibited by aqueous solutions of alkanolamines result in better surface wetting of pigment particles in paint and paint pigment grinds. Some liquid/liquid interfacial tensions6 are summarized in Figure 2.
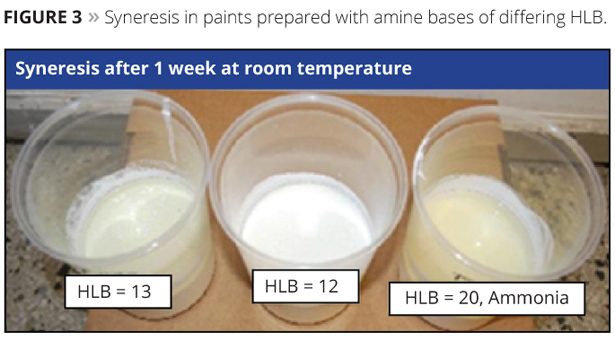
The lower liquid/liquid interfacial tensions exhibited by aqueous solutions of alkanolamines result in better pigment dispersion, emulsion stability and syneresis control in paints. Figure 3 demonstrates the impact that the HLB (calculated HLB using the formula discussed above) can have on emulsion stability in paint. Neutralizing agents with an HLB in the range of 9 to 12 are optimal for long-lived and stable emulsions. Paint formulas used in the demonstration are shown in Table 2 along with quantities in pounds per 100 gallons of paint. The quantity of amine used is approximately 12 pounds per 100 gallons with final adjustment to a pH ≈9.
Viscosity Stability
The viscosity stability of paints prepared with sodium hydroxide is often less than that of comparable amine-neutralized paints. Consider the three comparative paint formulas described in Table 3.
The performance properties of these three paints are summarized in Table 4. The viscosity of the paint prepared with caustic decreases more rapidly with heat age than identical paints wherein an alkanolamine is used in place of an inorganic base.
Viscosity stability issues are likely due to the different type of buffering that occurs when NaOH(aq) or KOH(aq) are used as the neutralizing base. Hydroxide is the conjugate base of water, and water has a pKa value of ≈14 @ RT. Alkanolamines are the conjugate bases of the corresponding ammonium ions, and typical alkanolamines used in paint have pKa values in the range of 9 to 11. The Henderson-Hasselbalch equation can be used to show the relationship between the pH of a solution and the ratio of protonated to unprotonated compound so long as the pKa of the compound is known

At a pH equal to the pKa of an acid, the acid is half protonated and half deprotonated. Paints are typically formulated to a pH between 8.5 and 9.5; in the center of the range of approximate pKa values for most alkanolamines but well below the pKa of water. Alkanolamines, in typical paints, exist as half protonated and half freebase (i.e., unprotonated), while hydroxide is almost completely protonated at a pH ≈9. Paints neutralized with hydroxide are buffered mostly by the conjugate bases of whatever other acids are present (e.g., polyacrylic acid, resin carboxylate groups, etc.). Trace acid produced by ester hydrolysis and/or microbial processes in a caustic-neutralized paint will quickly consume the trace of strong base present, and the caustic-neutralized paint will quickly end up being buffered by another system; typically a carboxylate and carboxylic acid pair, with the buffer pH dropping to ≈ 6 or lower. Paints neutralized by alkanolamines will consume significantly more acid before dropping to a given low pH value, as the alkanolamines are buffering at the “natural” pH of the formulated product. Viscosity stability can be markedly impacted by the increased strength of the excess hydroxide initially present but rapidly depleted by adventitious acid formation.
Conclusions
The primary deleterious effect of using caustic soda and/or caustic potash as a neutralizing base in paint results from an inherent decrease in colloid stability. Beyond this, the differing buffer properties of hydroxide-neutralized systems have an impact. The colloidal systems present within paint (emulsions, pigment dispersions, etc.) are less stable within hydroxide-neutralized formulas. In addition, the slight excess of hydroxide present in caustic-neutralized paints accelerates ester hydrolysis, and the use of strong bases can lead to poor long-term buffering.
References
- Busche, E.; Paints & Coatings Industry2012, April.
- Weiner, N. D.; Shah, A. K.; Kanig, J. L.; Felmeister, A.; J. Soc. Cosmetic Chemists1969, 20, 215-223.
- Z. Li, B. C.-Y. Lu; Chemical Engineering Science2001, 56 2879-2888.
- Feldkamp, K.; Chemie Ing. Techn. 1969, 21, 1181-1183.
- King, H. H.; Hall, J. L.; Ware, G. C.; J. Amer. Chem. Soc.1930, 52, 5128-5135.
- Gernon, M. D.; Alford, D.; Dowling, C. M.; Franco, G. P.; Tribology Transactions2009, 52(3), 405-414.










Report Abusive Comment