A novel line of air-drying resins has recently been introduced that will aid formulators of solventborne coatings meet increasingly restrictive VOC regulations. Known as oxidizable phenolic-based urethanes, or OPUs, these products can be used to modify and improve the properties obtained from conventional solventborne systems, including reduced dry time, enhanced toughness, mar resistance, high gloss and improved chemical resistance.
This article reviews the chemistry of traditional oxidizable vehicles, phenolic resins and urethanes in order to understand how this new resin technology transforms all three to produce improved results in the application and performance of VOC-compliant coatings.
Chemistry
In one hybrid molecule, oxidizable phenolic-based urethanes combine the chemistries of straight chain, unsaturated oxidizable hydrocarbons along with the cyclic rings of phenolics, and the contribution of urethane chemistry. Generically, the molecule can be represented as is shown in Figure 1.
The highly unsaturated hydrocarbon pendant chains allow drying, via oxidation, similar to other oxidizable vehicles such as drying oils, alkyds, oil-modified urethanes and oleoresinous varnishes. The phenolic portion contributes to quick film hardness, improved gloss, thermal stability, and excellent chemical, solvent and moisture resistance. The urethane chemistry provides a unique combination of toughness, abrasion, scratch and mar resistance, and an outstanding balance of hardness and flexibility.
A review of the chemistry, application and properties of oxidizable vehicles, phenolic resins and polyurethanes will aid our appreciation of how OPUs contribute to coatings.
Oxidizable Vehicles
Oxidizable vehicles include drying oils, alkyd resins, oil-modified urethanes and uralkyds. To appreciate how these vehicles cure, it is helpful to review their chemistry and their propensity to oxidize.
Drying Oils
Drying oils used for coating application are naturally occurring materials and are broadly classified as vegetable oils and marine oils. They include highly unsaturated vegetable oils (linseed, tung, etc.) and cooked vegetable oils, which are pre-polymerized or pre-oxidized, semi-drying oils (soya, sunflower, etc). Drying oils are still in use as film formers to produce household paints for indoor applications.
Chemically, all oils are triglycerides – compounds of one molecule of glycerin and three molecules of long-chain fatty acids. Fats are also triglycerides and differ from oils in that they are solids rather than liquids at room temperature. The various oils differ greatly in drying properties and other characteristics, depending on the kind of fatty acids contained.
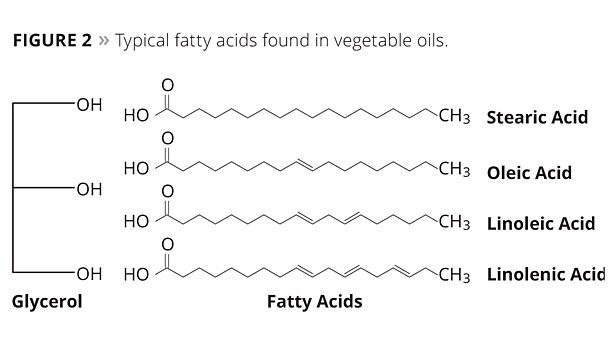
Glycerin, or glycerol, is a trihydric alcohol. The predominant fatty acids found in vegetable oils are 18 carbons in length with varying degrees of unsaturation. They include stearic, oleic, linoleic and linolenic (Figure 2). Glycerol combined with various fatty acids produces triglycerides having the generic structure shown in Figure 3.
The double bonds in the unsaturated fatty acids are chemically reactive sites. They are the points at which oxygen reacts with the oil to produce drying. Consequently, oils containing saturated acids (i.e., stearic) are nondrying (coconut, cottonseed, etc.), while oils containing acids with three double bonds (linolenic) dry most rapidly (linseed, safflower, etc). All natural oils contain mixtures of various fatty acids. Hence, the drying and other properties of an oil are determined by the kinds of fatty acids it contains and their amounts.
The fatty acids discussed above are predominantly found in oils such as linseed, safflower, soya, cottonseed, coconut and tall oil. The double bonds are isolated on separate carbon atoms. On the other hand, the fatty acids found in oils such as tung (China wood oil), oiticica and dehydrated castor oil contain double bonds on adjacent carbons in sequence, which makes them more reactive.
These double bonds are conjugated and allow faster drying and heat polymerization (heat bodying) properties. Because of their better drying properties, tung, oiticica and dehydrated castor oils are classified as hard oils, whereas linseed, safflower, soya, cottonseed and coconut are known as soft oils.
Licanic acid differs from eleostearic acid only in that two hydrogens in the chain are replaced by an oxygen, creating a keto or carbonyl group. The acid that distinguishes castor oil is ricinoleic acid. Like oleic acid, it contains only one double bond and accordingly, is non-drying. The difference is that a hydrogen is replaced by a hydroxyl group, and the presence of the OH group is key to dehydration and conjugation to make a valuable drying oil (Figure 4). When castor oil is heat treated with mineral acids or acid salts, water is split off at the OH group. This results in the removal of both the OH group and a hydrogen atom and the introduction of a second double bond that is conjugated relative to the original double bond. Consequently, drying properties are achieved.
The drying of an oil is a conversion from a liquid to a solid. When a drying oil is exposed to air in a thin film, there is a variable induction period during which oxygen absorption is negligible and there is no polymerization, which would be evidenced by thickening. The explanation is that the oil contains natural antioxidants, and they must be destroyed by oxygen before normal oxidation can commence.
Following the induction period, the oil absorbs oxygen from the air. Concurrently, there is a build up in the oil of unstable peroxide and hydroperoxide compounds. Polymerization starts with the development of the peroxides and is most rapid at the time when the peroxides are disappearing the fastest, indicating that decomposition of the oil-peroxide compounds introduces crosslinkage of the oil. Since the fatty acid chains that are crosslinked are in different planes, the resulting polymers are three dimensional, and the dried films contain ether linkages (-O-). The polymerization is accompanied by evolution of water, hydrogen peroxide and carbon dioxide.
It may be helpful to clarify the process of drying in stages of oxidation, polymerization, gelation and consolidation. Oils dry slowly without the use of metallic driers. Therefore, it is conventional to use driers to achieve practical drying times. Drier metals initiate the formation of free radicals from the unsaturated oil, which causes a rapid buildup of peroxides. The main role of the multivalent drier metal is to decompose these peroxides and regenerate the free radicals. The oxidized metals, in turn, are spontaneously reduced to regain their original form and, therefore, are catalysts in the true sense.
The important driers are metallic soaps of cobalt, manganese, calcium and zirconium. Although the napthanates are generally used, octoates (2-ethylhexoates) and tallates are also employed. As a rule, the metals are not used singly, but in combinations of two or three for synergistic reasons. Cobalt promotes rapid surface or top drying, but poor through drying. Manganese is similar to cobalt in effect but weaker. When maximum paleness and non-yellowing are essential, cobalt is used alone or with zirconium.
Alkyds
Alkyds are a class of polyester resins derived from the reaction of an alcohol and an acid or acid anhydride; hence the term alk-yd from “alcohol and acid.” They are typically manufactured from acid anhydrides such as phthalic anhydride or maleic anhydride, and polyols such as glycerine or pentaerythritol, and are modified with unsaturated fatty acids, mainly unsaturated C18 from vegetable oils, to give them air-drying properties. Modifying fatty acids come from vegetable oils such as soya, sunflower, coconut, dehydrated castor, linseed, tung and tall oil (resinous oil by-product from pulp and paper manufacturing).
As with drying oils, the unsaturated oils within alkyds react with oxygen from the atmosphere, which cause the oils to polymerize or crosslink with each other. The drying speed of the coating depends on the amount and type of drying oil employed. The more unsaturated the oil, the faster the reaction with oxygen.
Alkyd resins are produced via two processes – the fatty acid process and the alcoholysis process. Higher-quality, higher-performance alkyds are produced in the fatty acid process where the composition of the resulting resin can be more precisely controlled. In this process, an acid anhydride, a polyol and an unsaturated fatty acid are combined and cooked together until the final product has achieved a predetermined level of viscosity as suitable for its intended use.
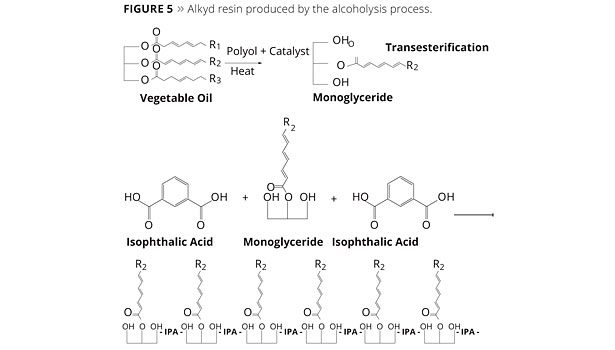
More economical alkyd resins are produced from the alcoholysis process where the end product quality control is not as paramount. In this process, raw vegetable oil, high in unsaturation, is combined with additional polyol and heated to cause transesterification of the triglycerides into a mixture of mono- and di-glyceride oils. To this resulting mixture acid anhydride is added to build molecular weight of the resin into roughly the same product as in the fatty acid process. However, the alcoholysis process produces a more randomly oriented structure (Figure 5).
In both cases, the resulting product is a polyester resin to which pendant drying oil groups are attached. At the conclusion of both processes, the resin is purified, diluted in solvent, and sold to paint and varnish makers.
Depending on the oil length (% oil), alkyds are commonly classified into three groups, which represent the relative fraction of drying oil component in the resin.
Long oil alkyds (60-85% oil length, 10-30% phthalic anhydride) are prepared from drying and semi-drying oils; pentaerythritol is the most recommended polyol. These resins are soluble in non-polar aliphatic solvents, are air drying and form glossy, moderately hard, flexible films. They are principally sold as medium-duty coatings to the consumer market and in some industrial coatings.
Medium oil alkyds (50-60% oil length, 30-40% phthalic anhydride) have less drying oil (prepared mainly with linseed and soya oils) and have a higher percentage of large-molecular-weight polyester backbone. These resins are soluble in aliphatic/aromatic solvent blends, are air drying, have improved mechanical properties and weather resistance, and are used in anticorrosive primers, maintenance paints and in road-marking paints in conjunction with chlorinated rubber. They dry more slowly and are also employed as high-gloss coatings and wood finishes. These resins can be made “water-thinnable” by using multifunctional trimellitic anhydride and dimethylol propionic acid, which is subsequently neutralized with an amine. Such resins have relatively low molecular weight and can be used for metal priming.
Short oil alkyds (30-50% oil length, 40-50% phthalic anhydride) will not air dry or harden unless heated. They are high-gloss, hard resins, soluble mainly in aromatic solvents. They are employed as baking enamels for finished metal products mainly in combination with phenol- and amino-formaldehyde resins.
Besides the fatty acids of vegetable oils, alkyd resins are modified with styrene, acrylates, isocyanates and polyamides. Styrene-modified alkyd resins find use in higher-solids paints, especially where high drying speed is required, as in maintenance coatings. Acrylic-modified alkyds have improved weather resistance. Uralkyds (alkyd modified with diisocyanate) are film formers for high-quality wood finishes. Polyamide-modified alkyd resins find application in thixotropic decorative paints for thick-layer application by brush.
In oil-modified urethanes, the alcoholysis of oil with glycols (trimethylol propane) produces monoglycerides (2 OH groups) and diglycerides (1 OH group). Generally, about 20% monoglycerides and 80% diglycerides are produced. The monoglycerides are chain extenders when reacted with diisocyanates.
Phenolic Resins
The earliest commercial synthetic resin was based on a phenol formaldehyde resin with the commercial name Bakelite, and was formed from the reaction of phenol with formaldehyde. These resins belong to the class of condensation polymers. In the formation of such polymers, two or more ingredients react to form a polymer and, in the process, parts of the molecules are eliminated as a by-product. In the case of most phenolic resins, the reactants are phenolic materials (phenol and substituted phenols) and aldehydic materials; water is eliminated.
Phenol, formaldehyde, water and catalyst are mixed in the desired amount, depending on the resin to be formed, and are then heated. The first part of the reaction, at around 70 °C, forms hydroxymethyl phenols. Phenol formaldehyde resins are formed by a step-growth poly-merization reaction, which may be either acid or base catalyzed. The pathway the reaction follows varies depending on the catalyst type used.
In the simplest case, the first-step reaction of phenol with formaldehyde is a simple addition reaction (Figure 6). Phenol can react with formaldehyde at any one of three possible sites at the ortho and para positions (sites 2, 4, and 6), allowing up to three units of formaldehyde to attach to the ring. Formaldehyde can react with up to two phenols. Thus, the theoretical functionality of phenol is three, the theoretical functionality of formaldehyde is two, and the actual functionality that is found in the polymer depends upon the phenol to formaldehyde ratio.
Once the phenol monoalcohol (hydroxymethylphenol) is formed, the course of the reaction depends on the reaction conditions, particularly on the pH. What happens is very different in an acidic system than in an alkaline one and, thus, it is usually convenient to consider them as two different types of resins.
Hydroxymethylphenol is capable of reacting with either another free ortho or para site, or with another hydroxymethyl group. The first reaction forms a methylene bridge, and the second forms an ether bridge (Figure 7).
The reaction that predominates will depend on the particular conditions involved. As the reaction continues and larger molecules are formed, the product of reaction becomes an exceedingly complex mixture. There is an increase in viscosity, and what was originally a low-viscosity liquid can become a solid.
In acidic systems, phenol alcohols are probably still formed, as shown in reaction 1. However, they condense so rapidly in reaction 2 that they cannot be isolated from the reaction mixture. By adding a small amount of acid catalyst to phenol (something miscible, such as PTSA) and slowly adding formaldehyde, the formaldehyde will react between two phenols to form a methylene bridge, creating a dimer. This dimer is contained in bisphenol F, which is itself an important monomer in the production of epoxy resins.
At higher concentrations of these dimers, there is the possibility of generating trimers, tetramers and higher oligomers. This is what occurs during the formation of a Novolac. The average molecule generated depends on the ratio of formaldehyde to phenol. In Novolacs, this is usually around 0.8 and, thus, with five phenols for every four formaldehydes, the average molecule is a pentamer. As a result, large molecules consisting of phenolic nuclei linked by methylene bridges build up rapidly. The viscosity of the reaction mixture increases rapidly, and a solid resin is soon formed.
Thus, acid-catalyzed phenol formaldehyde resins are made with a molar ratio of formaldehyde to phenol of less than one (0.7 to 0.9) and are referred to as thermoplastic, Novolac or two-step resins. In the coatings industry they are generally referred to as non-heat-hardening or non-heat-reactive resins. Novolacs are soluble in alcohol and are useful as coating modifiers and commonly used as photoresists. Owing to the molar ratio of formaldehyde to phenol, they will not completely polymerize without the addition of a crosslinking agent. A common crosslinker used for Novolac is paraformaldehyde.
In strongly alkaline systems, additional formaldehyde may add on to the hydroxymethylphenol to give phenol di- or tri-alcohols. These base-catalyzed phenolic resins are made with a formaldehyde-to-phenol ratio of greater than 1 (1.0 to 3.0). If the reaction temperature is low enough and reaction time short, the product may consist almost entirely of a mixture of the various possible phenol alcohols. However, at higher temperatures, or if the reaction time is extended, the phenol alcohols will condense either with unreacted phenol or with other phenol alcohols.
Hydroxymethyl phenols will crosslink on heating to around 120 °C to form methylene and methyl ether bridges. At this point the resin is starting to crosslink, to form the highly extended three-dimensional web of covalent bonds, which is typical of polymerized phenolic resins. It is this highly crosslinked nature of phenolics that gives them their hardness, thermal stability and that makes them impervious to most chemical attack and solvation.
When ammonia or amines are used as the catalyst, the ratio of moles of combined formaldehyde to moles of phenol is usually around 0.9 to 1.5. The resulting finished resin is somewhere between the strongly alkaline and the acidic one. Phenol alcohol groups are present in the product at all stages, but large molecules form quite rapidly, and the product is never as low in viscosity as when a strong alkali is used. It becomes a solid more readily. Moreover, much of the catalyst becomes an integral part of the resin. CH2OH groups may be replaced by CH2NH2 groups and CH2-O-CH2 bridges by CH2-NH-CH2 bridges.
In many of the industries where phenolic resins are used, those resins produced with alkaline catalysts, either strong or weak, are referred to as thermosetting, resole or one-step resins. In the coatings industry they are generally referred to as heat-hardening or heat-reactive. They self condense on heating thanks to the reaction of methyl-ol groups to give an insoluble crosslinked polymer having a sufficient hardness.
In the synthesis of phenolic resins, it is helpful to review the functionality of the reactants, as that will determine the ultimate properties of the end product. The functionality of the aldehyde is two, while that of the phenol depends on the particular phenol used. The potentially reactive positions in a phenol are the unoccupied positions ortho or para to the phenolic hydroxyl. Phenol itself, in which all three positions are unoccupied, has a functionality of three: o-cresol, in which one ortho position is occupied, has a functionality of two; 2-6 xylenol has a functionality of one; and catechol, with two hydroxyls and no other substituents, has a functionality of four.
When the phenol functionality is one, reaction with an aldehyde gives a simple chemical compound, not a resin. When it is two, the reaction gives a resin, but a resin without any three-dimensional crosslinking and, therefore, a resin that is permanently fusible and soluble. When the functionality is more than two, the resins formed can crosslink to give insoluble and infusible products. It is important, therefore, to select the phenol to use in accordance with the properties required in the end product.
Polyurethanes
Polyurethanes are the most prolific family of high-quality film formers. The chemistry is based on the reaction between a di- or polyisocyanate monomer, or prepolymer, and a di- or polyhydroxyl prepolymer. The unique combination of the properties of toughness, outstanding balance of hardness and flexibility, abrasion, scratch and mar resistance, solvent resistance, high gloss and clarity, plus the ability to be “custom tailored,” has led to urethanes’ widespread use. End-use applications include leather coatings, fabric finishes and adhesives, industrial maintenance and corrosion-resistant coatings, floor varnishes, seamless flooring, marine coatings, magnet wire coatings and concrete sealers.
When aliphatic isocyanates like IPDI and HDI are used as monomers, polyurethane resins exhibit excellent weather resistance and are suitable for use in automotive clearcoats. Produced with aromatic isocyanates like TDI and MDI, polyurethane resins possess excellent chemical and heat resistance, but tend to yellow when exposed to outdoor conditions.
Several chemical reactions enter into both the formation and curing of urethane coatings. Foremost in resin manufacture, and also useful in film forming, is the reaction of an isocyanate group with hydroxyl groups present in polyesters, polyethers, alcoholated drying oils and castor oils. Polymer formation is made possible by using di- or polyfunctional isocyanates and hydroxyl-terminated compounds. Typical is the reaction between 2,4 toluene diisocyanate (TDI) and a polyether, such as polypropylene glycol, to form an isocyanate-terminated polyurethane (Figure 8). Commercially available polyethers have molecular weights ranging from a few hundred to several thousand.
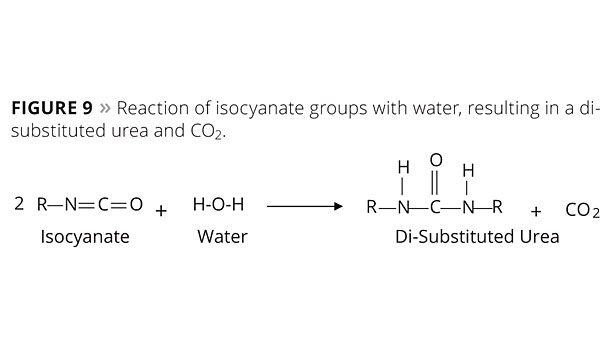
The next most important reaction is that of isocyanate groups with water, resulting in a di-substituted urea and CO2 (Figure 9). This is the mechanism of all moisture curing urethanes and it enters into the cure of two-package systems to varying degrees.
Isocyanates are water-sensitive monomers that eagerly react with water, producing urea groups and CO2. Therefore, isocyanate-containing formulations require the incorporation of moisture-free components (solvents, pigments and additives) as well as the addition of moisture scavengers. Hydroxyl-containing solvents should be avoided.
The reaction shown previously produced linear polyurethanes. Practical coatings, however, contain varying amounts of crosslinks, which are introduced in two ways. Most important is the use of higher-functional polyols, commonly triols, to create a specific degree of crosslinking per weight of molecule. A higher number of crosslinks produce harder, less-flexible coatings.
Crosslinking is also produced by the reaction of terminal isocyanate groups in the prepolymer with urethane linkages to form an allophanate (Figure 10). Or, the reaction of the isocyanate with the urea groups introduced by the water reaction to form a biuret (Figure 11).
Urethane Classifications
Urethane coatings are commonly categorized as belonging to one of five classifications that are chiefly related to the curing mechanism.
Oil Modified
These are reaction products of isocyanates and alcoholysis products of drying oils. The process is analogous to that used in alkyd manufacture and, as with alkyds, produces an upgraded drying oil. Curing is accomplished by oxidation of the unsaturated oil.
Moisture Cure
Isocyanates are polymerized with diols and triols such as polyethers, polyhydric alcohols and castor oil products. These prepolymers contain some stoichiometric excess of isocyanate groups (unreacted terminal isocyanate groups) by design. The groups react promptly with atmospheric moisture, producing urea groups that further crosslink the prepolymer by reacting with the rest of the isocyanate functionalities.
Two Component
Two component (2K or two-pack) polyurethane film formers are based on the use of liquid diisocyanate and poly-hydroxyl monomers with a minimum of organic solvent added to the hydroxyl component. Film formation is based on forming a crosslinking reaction between components, mixed immediately before application, or inside a specially designed spray gun.
Isocyanates are reacted with relatively low-molecular-weight polyols such as alcohols to form “adducts.” These adducts then form one part of a two-part system; chain extension and curing are obtained from the second component, which can be any of the polyols referred to earlier.
Blocked
This is a variation of the two-component system. Isocyanate groups can form reversible adducts with suitable monohydroxyl compounds such as phenol. Thus, the adduct is “blocked,” making it unreactive at room temperature, which then allows it to coexist with hydroxyl-containing prepolymers is a single pack. Further, the components can be packaged in one can with pigments and additives. Deblocking occurs on heating (above 120 °C), which frees the isocyanate groups to react with the polyol.
Prepolymer Plus Catalyst
These are essentially the same as moisture-cured urethanes but are provided with a separate catalyst to accelerate cure.
Oxidizable Phenolic-Based Urethanes
A review of the chemistry, application and properties of oxidizable vehicles, phenolic resins and polyurethanes was essential to appreciate how OPUs contribute to coatings. These resins are capable of exhibiting the benefits afforded by all three in one hybrid molecule.
The highly unsaturated hydrocarbon side chains in OPUs allow them to cure like (oxidize), and be compatible with, drying oils, alkyds, oil-modified urethanes and oleoresinous varnishes. With the appropriate drier combination, set time, tack-free time and dry time is improved (Figure 12).
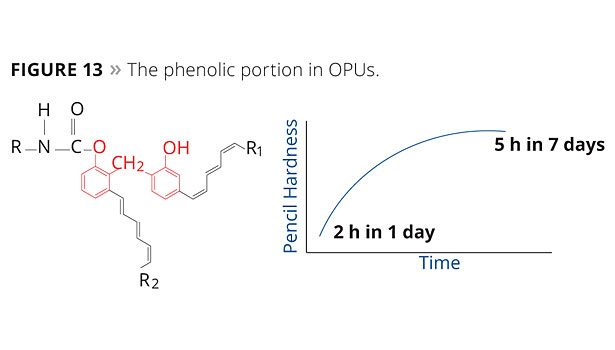
The phenolic portion in OPUs contributes to quick film hardness, improved gloss, thermal stability, and excellent chemical, solvent and moisture resistance (Figure 13).
The urethane chemistry within OPUs contributes a unique combination of toughness, abrasion, scratch and mar resistance, clarity, and an outstanding balance of hardness and flexibility. Further, the addition of the urethane’s polarity, as well as the unsaturated hydrocarbon side chains, allow these products to be compatible, and readily diluted, with both oxygenated and non-polar solvents (Figure 14).
Conclusion
The unique hybrid chemistry of oxidizable phenolic-based urethanes combines the drying property of fast-drying oeloresinous vehicles, the chemical resistance of phenolics and the toughness of urethanes. As a modifying resin for vegetable oils, alkyds, oil-modified urethanes or uralkyds, a coatings formulator can expect to achieve low-VOC, low-viscosity, fast-drying, and super-hard finishes for a myriad of wood applications including varnishes and stains. n
This paper was presented at the Eastern Coatings Show, April 2013, in Atlantic City, NJ.
For more information, visit www.spmorell.com.













Report Abusive Comment