UV-curable polyurethane dispersions (UV-PUDs) are a well-established and commercially successful class of products because of their combination of low environmental impact, range of achievable properties and ease of use. Important end-use applications for UV-PUDs include factory-applied wood coatings, field-applied coatings on concrete, wood or vinyl flooring, and protective coatings on plastics. All of these applications require high degrees of hardness plus scratch and abrasion resistance. PUDs made from renewable raw materials are also well known (including some UV-curable varieties), but are generally based on vegetable oil derivatives and, even after UV curing, are too soft for the applications mentioned above. This article discusses unique new UV-PUDs containing a high percentage of renewable carbon that have properties suitable for use in high-performance coatings.
Introduction
UV-PUDs combine attractive aspects of two environmentally friendly coating technologies – waterborne and UV curing. In addition to the sustainability aspects, UV-PUDs deliver a unique combination of performance attributes that combine some of the best features of conventional waterborne and 100% solids UV coatings.1-3 The sustainability and performance of UV-PUDs has led to their commercial success and high market growth rates over the past several years. Use of renewable or biobased raw materials in UV-PUDs can bring another level of sustainability to this technology. There are many reports of UV-PUDs partially made from renewable material,4-7 but many of these are based on vegetable oil derivatives, with the final coatings being relatively soft and lacking some properties needed for high-performance coatings such as hardness, water resistance, stain resistance and scratch and abrasion resistance.
In the past several years there has been an explosion in the commercial availability of chemical building blocks derived from renewable resources, and renewed interest in application of others that have been long been items of commerce.8 Nearly all of the renewable raw materials used commercially are plant based and are converted to industrially useful raw materials using a range of chemical processes such as esterification, epoxidation, hydrogenation, fermentation, hydrolysis, pyrolysis, dehydration and polymerization. Taken together these processes have given rise to the concept of the biorefinery.9
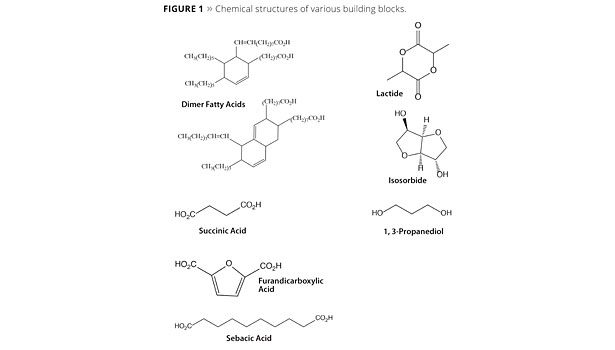
A number of these building blocks are renewable versions of molecules that have traditionally been made from petrochemicals. Processes for production of succinic acid, 1,4-BDO, 1,3-propanediol, propylene glycol, adipic acid and hexamethylene diamine among others are already commercial or in development. Many other useful building blocks are unique structures that are only practically available from renewable sources such as vegetable oils and their derivatives including unsaturated fatty acids, C36 and C54 dimer and trimer products, 1,4:3,6 dianhydrohexitols such as isosorbide, and furan derivatives such as 2,5-furandicarboxylic acid. Some example structures of both types are shown in Figure 1.
We have developed an approach to incorporate significant renewable content into high-performance UV-PUDs based on the use of polyester biopolyols. By combining the appropriate monomer building blocks, biopolyols can be designed that bring the hardness, abrasion resistance and other properties required for many UV-PUD-based coatings applications.
Results and Discussion
A series of polyester biopolyols with 100% biocarbon content was prepared from various combinations of raw materials selected from those described above using standard synthesis methods. After screening several with a range of compositions, molecular weights and physical properties, two were chosen for this study. Choices were made based on the relative ease of preparation, ready availability of the raw materials and expected performance in UV-PUD-based coatings. As these polyester biopolyols are designed to be quite hard, and UV-PUDs derived from them tend to be brittle, we found it advantageous to use them in combination with a more flexible biopolyol. Cerenol 1000 (DuPont) is a 1000 molecular weight poly(1,3-propanediol) polyether derived from DuPont’s Susterra bio-sourced 1,3-propanediol.10 Properties of these biopolyols are shown in Table 1.
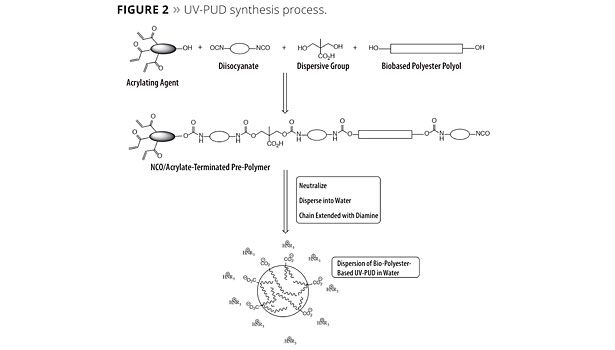
UV-PUDs were prepared from the polyols in Table 1 using a process based on classical methods but with modifications developed in our labs. The overall process, which is shown schematically in Figure 2, involves three essential steps: 1) formation of an acrylate/isocyanate-terminated pre-polymer; 2) neutralization of the carboxylic acid group of the prepolymer with a tertiary amine; and 3) reaction of the remaining NCO groups with a diamine to chain extend the polymer. The pre-polymer is made from a multifunctional acrylating agent, a di- or polyisocyanate, a dispersive agent (in this case dimethylolpropionic acid) and one or more biopolyols. As prepared by our method, the final dispersions are completely solvent free and contain no added surfactants or tin catalysts.
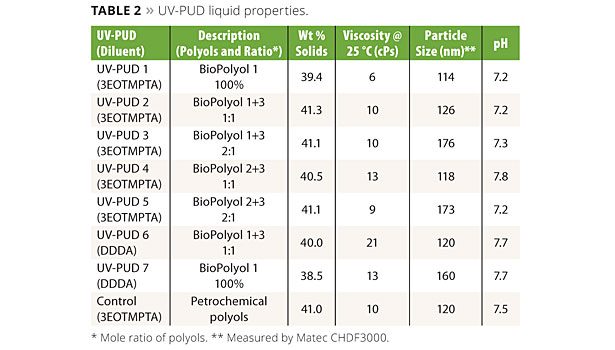
Liquid properties of the UV-PUDs made by this method are shown in Table 2. The Control UV-PUD shown in the table is a product prepared entirely from petrochemical-sourced raw materials; it was developed in our labs for use in high-end wood coatings and will be commercialized in the near future. The properties of the biopolyol-based UV-PUDs are compared to this control product throughout this article. All of the products in Table 2 have good dispersion stability in room temperature storage, 1 h centrifuge and 60 °C four-day stability tests as measured by visual observation for separation and monitoring of viscosity and particle size.
As is typical of UV-PUDs, all of the products contain some acrylate monomer reactive diluent as an integral part of the composition. The reactive diluent helps to lower the MFFT of the system, promote particle coalescence and also contributes to cured coating properties. UV-PUDs 1 - 5 and the Control contain 3 mole ethoxylated trimethylol-propane triacrylate (3EOTMPTA, Sartomer SR454) as the diluent. UV-PUDs 6 and 7 contain 1,10-decanediol diacrylate (DDDA, Sartomer CD595), which is a biobased reactive diluent made from a castor oil derivative. All of the UV-PUDs in the table show good water release behavior, have MFFTs of less than 0 °C and form tack-free films after physical drying, but before UV curing.
For curing and testing of the coating properties, the UV-PUDs were incorporated into a simple formulation consisting of 63.5% UV-PUD (after adjusting to 35% solids) plus 1% associative thickener, 0.5% leveling agent and 5% Irgacure 500 photoinitiator based on solids. Drawdowns were made at 6 mils wet film thickness on Leneta charts, glass plates or aluminum panels, depending on the tests being run. To ensure water was completely removed from the films and not affecting the test results, samples were dried for 30 min at 25 °C and 30 min at 60 °C (tests done after drying 10 min at 25 °C and 10 min at 60 °C showed similar results to those below). The dried films were cured with 410 mJ/cm2 UVA energy by running at 50 fpm through an Inpro UV cure unit with two Hg lamps.
Results from testing of the cured films are shown in Table 3. All of the products have very good hot water resistance and good chemical resistance passing 200 MEK double rubs without damage. A few trends can be discerned from the data.
Overall the performance of the biopolyol-based UV-PUDs approaches that of the Control in most aspects, with the exception of stain resistance. Most of the deficiency is in mustard stain resistance and can be attributed to a structural moiety in the Control that has no biobased replacement and that we know from other work improves mustard staining.
Incorporation of a certain amount of the polyether BioPolyol 3 in the pre-polymer is effective in flexibilizing the cured coating. Although the hardness is reduced, the Taber abrasion resistance actually improves somewhat in the more flexible coatings. This is not an uncommon observation, as a tougher, more resilient coating is better able to resist fracturing or flaking off under the test conditions. In all cases, addition of the polyether biopolyol is detrimental to stain resistance.
Comparison of the results for the pairs of UV-PUDs 1 vs. 7 and 2 vs. 6 allows comparison of the effects of the reactive diluent on coating properties. UV-PUDs 1 and 2 both contain trifunctional 3EOTMPTA as the reactive diluent, while UV-PUDs 2 and 6 contain the biobased difunctional 1,10-DDDA. Use of the difunctional reactive diluent results in a slight decrease in hardness and stain resistance, but an improvement in flexibility and little to no change in Taber abrasion resistance. It is apparent that the UV-PUD itself is contributing the bulk of the coating properties.
By comparing UV-PUD 2 vs. 3 and 4 vs. 5 it can be seen that use of Biopolyol 1 results in cured coatings that are slightly softer and more flexible than those made from Biopolyol 2. This is understandable based on the biopolyol structures, and the results give insight that can be used to further tailor and optimize properties.
Conclusion
By using carefully selected renewable building block monomers, we can design polyester biopolyols with 100% renewable carbon content that can be used to produce high-performance UV-PUDs suitable for use in many coatings applications. Properties of the UV-PUDs can be tailored by design of the biopolyols, using combinations of polyols or by choice of reactive diluent. Patent applications have been filed on this unique new technology. Work is continuing to further improve the performance of these products and better understand the range of potential applications for them.
References
1 Bernquist, H.; James, D.; Ekvall, K.; Wennerberg, P.; Sorenson, K. Novel Acrylated Building Blocks for UV-Curable Polyurethane Dispersions, Radtech Europe 07 Conference Proceedings.
2 Tielemans, M.; Roose, P.; Ngo, C.; Lazzaroni, R.; Leclere, P. Progress in Organic Coatings2012, 75, 560-568.
3 Tielemans, M.; Roose, P.; De Groote, P.; Vanovervelt, J-C. Progress in Organic Coatings2006, 55, 128-136.
4 Rengasamy, S.; Mannari, V. Progress in Organic Coatings2013, 76, 78-85.
5 Chang, C-W.; Lu, K-T. Progress in Organic Coatings2013, 76, 1024-1031.
6 Rengasamy, S.; Mannari, V. Progress in Organic Coatings2014, 77, 557-567.
7 Sommer, S.; Luehmann, E.; Flores, L.; WO2011/107398A1
8 Biron, M. The Highway Towards Bio-polymers: The Breakthrough of Biobased Monomers and Building Blocks, Oct. 9, 2013; http://www.specialchem4bio.com/articles/2013/10/bio-the-highway-towards-bio-polymers.
9 http://en.wikipedia.org/wiki/Biorefinery.
10http://www2.dupont.com/Cerenol_Polyols/en_US/ and http://duponttateandlyle.com/.
Acknowledgements
The authors would like to thank Bill Schaeffer and Steve Tyson of Sartomer Americas’ Technical Service and Development group for support and advice on curing and testing methods in support of this paper.
This paper was presented at the 2014 RadTech UV & EB Conference in Rosemont, IL.






Report Abusive Comment