Truly novel polymer platforms are few and far between, arising perhaps once in a generation. Epoxies, urethanes, polyesters, acrylics and others have long been industry mainstays, and each of these chemical platforms offers certain advantages, depending upon specific application needs. At the same time, new options offering significant energy savings or emitting lower levels of VOCs would be extremely useful.
Most coating systems contain either solvents or water, necessitating the use of ovens to cure or dry the coating, an energy-intensive process. The use of solvent also generates VOCs, raising regulatory and safety concerns. In many cases, conventional coatings can leach materials, such as non-reactive diluents, bisphenol A, formaldehyde and styrene, which may have unfavorable environmental or health effects. In addition, certain conventional coatings use corrosive or sensitizing materials, such as amines or isocyanates for their cure. A versatile, 100%-reactive, rapid room-temperature curing polymer system would provide a host of benefits not currently seen in any single technology to date.
A new polymer platform is bringing a new category of disubstituted alkenes – a unique group of monomers – to manufacturers’ doorsteps. This 100%-reactive class of materials requires no solvents, heat or ultraviolet light to cure, and should allow manufacturers to rethink how they approach a wide variety of applications. Such monomers, their derivatives and the resulting polymer platforms will enable rapid-cure, high-performance products that can be cured at ambient temperatures. As such, this new technology promises numerous product and processing improvements in the adhesives, coatings, automotive, printing and many other markets. Ambient curing will also expand the use of thermally sensitive substrates that were previously inaccessible to heat-cured adhesives and coatings. Finally, absence of solvents and non-reactive diluents will afford substantial environmental benefits.
A Familiar Manufacturing Riddle Solved
Chemists have long realized that electron-deficient disubstituted alkenes, with their ability to polymerize anionically, held great promise for adhesives, coatings and other applications – if only these chemicals could be stabilized during the synthetic process and subsequent applications. Development of a scalable process that could deliver commercially viable yields of these highly reactive monomers also remained a challenge.1-3
Now there is a way to reliably produce these highly reactive materials and bring them to market on an industrial scale. Proprietary stabilizers control the monomers’ reactivity, preventing degradation and spontaneous polymerization during manufacture, transport and storage.4 Catalysts (or in some cases, the substrate surface itself) are then used to initiate the polymerization reaction, which can occur ambiently as a react-on-demand process. While conventional free-radical initiators may also be utilized to activate this chemistry, using photo or thermal means of activation, this initiation method fails to take advantage of the high ambient reactivity enabled by anionic polymerization. Manufacturers have the freedom to choose the most appropriate catalyst method to ramp up production speeds and save time and energy while making the best use of existing processing technologies.
Customizable Functionality
Disubstituted alkene monomers have been made to coat steel, tin-plated steel, aluminum and glass faster than other technologies and/or with less energy. The potential benefits of these new platform monomers, after formulation, may include:
- Ambient anionic cure – no ovens needed;
- Applicability to substrates that can’t be heated;
- Chemical and heat resistance;
- Fast cure speed;
- Optical clarity;
- No blooming;
- Low viscosity.
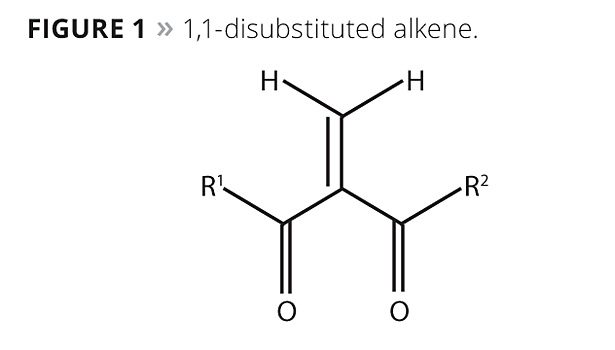
One such monomer class, offered under the trade name Chemilian™, consists of 1,1-disubstituted alkenes with a single, fast-curing alkene group and two terminal groups (Figure 1). These substituent terminal or functional groups may be the same (symmetrical) or different (asymmetrical), and can be selected to create polymers having diverse properties. In Figure 1, the properties of R1 and R2 will determine the final polymer characteristics such as flexibility, rigidity, crystallinity, hardness and degree of solvent or chemical resistance. The single, highly reactive alkene group (center) allows rapid polymerization at room temperature.
Researchers have only scratched the surface in exploring the potential for these monomers. Says Jeff Sullivan, “We are just starting to explore the possibilities.” Sample-sized batches of monomer are now being supplied to commercial partners who are beginning to conduct their own investigation into specific applications.
Multiple Alkene Groups for Crosslinking
Another available monomer class, having the trade name Forza™, features multiple alkene groups for crosslinking (Figure 2). The identities of R1 and R2 will affect the final polymer’s characteristics, while the two highly reactive methylene groups allow fast polymerization, even at room temperature. These monomers were created for developing high-strength polymers with higher stiffness and higher thermal, chemical and scratch resistance in coatings and adhesives as compared to the monofunctional monomers. The ability to crosslink this platform of monomers rapidly in ambient conditions distinguishes it from other anionic curing monomers like cyanoacrylates.
React-on-Demand or “Pre-Polymerized” Polymers
At this time, disubstituted alkene technologies can be formulated as react-on-demand or solvent-based coatings. The react-on-demand systems are either activated by the surface to which they are applied or, in instances where the substrate surface is not sufficient to initiate polymerization, by application of a secondary initiator. Cure times for these systems can be varied from a few seconds to several minutes, depending on application requirements, by varying the type of monomers used in the formulation or the type and concentration of initiators.
In addition, the monomers can be anionically or radically pre-polymerized in solvent and either isolated or formulated for specific coating applications. The polymerization characteristics depend on the type and concentration of initiator, the type of solvent and the nature of the monomer. The polymer’s molecular weight can be adjusted by terminating this reaction when the desired molecular weight has been reached. The resultant solvent-based polymer will then be ready to be compounded for use as a coating. Lab-scale experiments have demonstrated average molecular weights ranging from 10,000-300,000, depending upon the choice of initiators and solvents. Further formulation with various additives may be possible at this stage to improve properties of the resultant coating. Sirrus envisions one-part coating formulations in which the polymerization of disubstituted alkene monomers can occur rapidly in situ.
Untold Possibilities
So far, dozens of disubstituted alkene analogs have been developed, such as diethyl methylene malonate (Figure 3), exhibiting a wide range of utility. R groups can be esters, amides or other similar groups. Chemilian is a monomer where the R groups are esters with various side groups. Table 1 covers some of the basic coating and adhesive properties of those monomers. The majority of this work was conducted with unformulated monomers or blends of monomers; formulated systems are specified where used.
In adhesive applications, alkene monomers have demonstrated adhesion to polar plastics [e.g., polycarbonate, acrylonitrile butadiene styrene (ABS), glycol-modified polyethylene terephthalate (PETG), acrylics, high-impact polystyrene (HIPS), etc.] and soda-lime (window) glass without the introduction of additional catalyst. In these cases, the polymerization of the monomer is initiated by the presence of basic species in the substrates, and subsequent lap shear testing of resultant joints shows substrate failure in the plastic and glass adherends. For metals such as aluminum and steel, high bond strength can be achieved using an initiator on the surface prior to application of the monomer. Adhesion on porous substrates like wood requires a combination of rheology modification to prevent wicking of monomers, and polymerization through an appropriate secondary activator. Figures 4 and 5 summarize the adhesive performance of alkene monomers.
Potential Applications
Of the many promising coating applications for disubstituted alkenes, a few are particularly striking.
Bisphenol A (BPA)-Free Can Linings
Recent media coverage has questioned the safety of widely used food and beverage can coatings. BPA-based epoxy resin linings are thought to pose a potential risk to consumers. Coatings made with disubstituted alkenes, on the other hand, will not contain BPA. Furthermore, the promise of rapid cure can increase production speed and reduce heating costs.
Smart Coatings
Since disubstituted alkene monomers can be functionalized by incorporating various side groups that are bound to the backbone, and these entities can undergo living anionic polymerization, there is an extremely broad range of monomers that can be synthesized to facilitate smart coatings. For instance, surface modification of metals to produce water-repellent, corrosion-resistant or antimicrobial surfaces may be possible with this technology.
Composite Binders
The high reactivity of disubstituted alkene monomers makes them good candidates for use as composite binders. Monomers can be activated by the surfaces of glass fibers, beads and powders, as well as other basic ingredients, forming toughened, highly filled systems that can be used in insulation, encapsulation and potting applications. The use of high filler loadings can significantly reduce cost of the resultant composites, and activation by the filler’s surface allows excellent adhesion and fast processing.
Reactive Diluents
Disubstituted alkene monomers can be activated by the amine-based catalyst and activator systems often used in two-part epoxy adhesive and coating systems. The low viscosity of alkene monomers may enable their use as replacements for the reactive diluents in current use. Alkene monomers are made using a halogen-free process, are fast reacting, and will likely provide mechanical property synergies with the principal reactive oligomeric systems.
Conclusion
A new, 100%-reactive, energy-saving, solvent-free, low-VOC polymer platform for formulating coatings is now available. Rapid room-temperature curing disubstituted alkene monomers offer numerous possibilities for a wide variety of previously unfeasible, value-added adhesives and coatings that require no solvents, heat or ultraviolet light to cure. This new technology offers potentially significant changes and savings in manufacturing, assembly and raw materials, with greater environmental suitability and enabling the use of heat-sensitive substrates. It is expected that leaders in the adhesives, pigments and coatings industries will be eager to investigate the properties of formulated disubstituted alkene systems further. As Jeff Sullivan says, “Though still in its infancy, no one yet knows the potential that may be unlocked by this technology.”
References
1 US Patent number 2,330,033-D. Alelio Gaetano
2 US Patent number 3,221,745- Coover, et.al.
3 US Patent number 3,758,550- Eck, et.al.
4 US Patent number 8,609,885 B2- Malofsky, et.al.









Report Abusive Comment