The adhesion of reinforcing textiles in the rubber industry has long been achieved through various approaches, including the modification of the textile by primer coating and surface treatment. Many approaches have dealt with the modification of the rubber combined with complementary treatment of the textile fibers.[i],[ii] Resorcinol Formaldehyde Latex (RFL) treatment is still the most popular process to treat fiber for rubber applications. However, using RFL presents environmental and processing challenges during manufacturing steps. Most RFL treatments require a multi-step process, and its shelf life limits productivity and reproducibility of results.
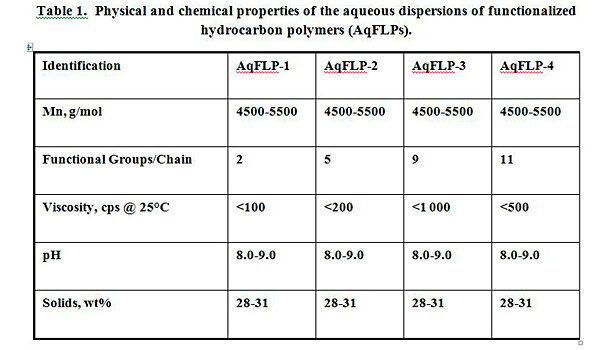
In the continuing effort to promote more environmentally friendly and easy-to-use products, aqueous dispersions of functionalized low-molecular-weight hydrocarbon polymers (AqFLPs) have been developed.[iii] Table 1 summarizes the physical and chemical properties of the dispersions. AqFLPs show good stability, low viscosity and small particle size. The development of these aqueous dispersions can allow for a wider range of waterborne applications including textile and other substrate treatment. They can also be used in a number of other surface modification areas, including adhesive and coating applications. When properly formulated into surface treatments, the dispersions can increase rubber adhesion to textile, metal and plastic substrates, improve chemical resistance, and affect more homogeneous dispersions of polar fillers in non-polar rubber and plastics.[iv] The hydrophobic and hydrophilic components of the dispersions allow for interaction between polar and non-polar substrates. With the addition of aqueous AqFLPs to styrene butadiene (SB) and styrene butadiene vinyl pyridine (VP) emulsions, excellent compatibility was observed and improvement in adhesion was demonstrated. The blends were homogeneous, grit free and showed good stability.
The present study will demonstrate the utility of the AqFLPs as a formulating tool to develop improved surface treatments for rubber-to-substrate adhesion. Adhesion performance as a function of reactive group content on the AqFLPs, type of latex in the formulation, vulcanization system, and substrate material will be outlined.
Experimental
Rubber Compound
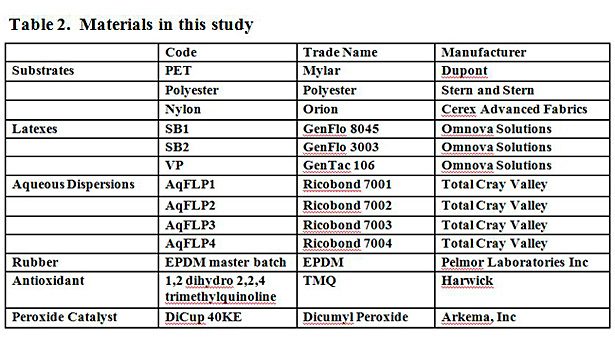
Table 2 shows the materials used in this study. A masterbatch of EPDM compounded rubber was supplied by Pelmor Laboratories Inc. The masterbatch contains 100 phr EPDM, 50 phr aromatic oil, 100 phr carbon black, and 1 phr stearic acid. 96.8 phr of the masterbatch was used and combined with 0.4 phr 1,2 dihydro 2,2,4 trimethylquinoline (antioxidant) and 2.8 part dicumyl peroxide (DiCup) 40 KE (peroxide catalyst). The ingredients were compounded on a 2-roll mill until made homogenous, and cure profiles were obtained for each batch to ensure uniformity using a PPA2000 (Alpha Technologies).
Substrate Treatment
AqFLPs and AqFLPs/latex blends were produced to evaluate the effect of their chemical composition on the overall adhesion performance. Direct adhesion testing (coating) and rubber adhesion of different substrates to peroxide cure EPDM rubber were evaluated.
A one-step dip process was used to treat the fabric substrates. Blends were made combining AqFLPs and three emulsions: SB1, SB2 and VP. The blends were made at two, latex: AqFLPs weight: weight ratios, 90:10 and 80:20. The final composition of the blends was 44% solids. Each fabric was dipped in a bath then passed through rollers to remove excess material. The treated fabric was then dried in an oven for 6 min at 150 °C. The PET film was coated as a draw-down using a #20 Mayer rod. The treated film was then dried in the oven for 1 min at 150 °C.
Physical Testing
Modified cross-hatch adhesion test
Using a razor blade, cuts in the form of a cross-hatch pattern were made into the coating. A pressure-sensitive tape was placed on top of the pattern and then removed quickly. The adhesion was then evaluated by the amount of coating removed and the degree of difficulty by which it was removed.
T-peel adhesion test
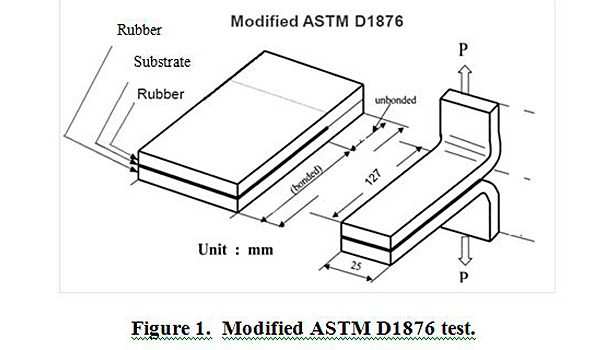
Plaques of rubber-fabric-rubber were made and cured at 160 °C for 35 min in a heated press, based on the cure profile of the EPDM rubber. These plaques were then cut into four 1-inch-wide by 5-inch-long strips. In the case of PET film, coated films were pressed onto uncured EPDM rubber and cured under the conditions mentioned above. A modified ASTM D1876 T-peel method was adapted to test the adhesion of the treated fabric to cured EPDM rubber. Figure 1 provides a visual description of the modified test. A Thwing-Albert EJA Vantage 10 tensile tester was used to perform the testing. T-peel speed and distance were constant for all samples. All strips were tested at room temperature (27 °C) and at 50% relative humidity.
Results and Discussion
Adhesion of AqFLPs-Coated PET Film
A cross-hatch method was used to test the adhesion of just the AqFLPs coated onto PET film. Testing of coatings containing AqFLP-1 and AqFLP-2 resulted in complete delamination of the cross-hatched coating from the film, while AqFLP-3 and AqFLP-4 failed at the tape-coating interface (no coating- PET film failure). The dried coatings showed very slight to no tack and had good flexibility.
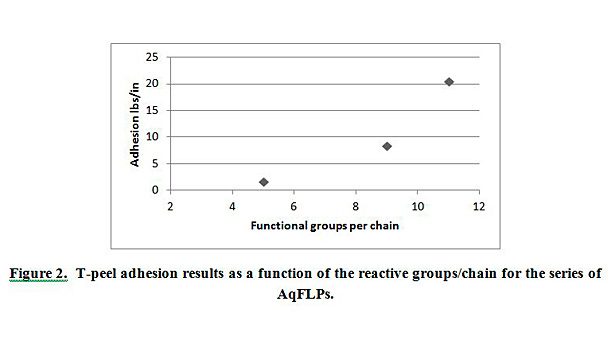
To further test the adhesion of these coatings, a T-peel adhesion method was used to test the adhesion between the PET film and EPDM peroxide-cured rubber. The PET film was treated using the dispersions AqFLP-2, AqFLP-3, and AqFLP-4. Figure 2 demonstrates that the adhesion values increased as the reactive group content of the AqFLP increases. AqFLP-4, containing 11 functional groups per chain, shows the best adhesion. In fact, the failure mechanism for this treatment was cohesive and the recorded value represents the cohesive strength of the rubber.
Adhesion of AqFLP/Latex Blends-Coated Substrates
Blends were made using AqFLPs and the three latexes SB1, SB2 and VP. The blends were made at 90:10 and 80:20 latex: AqFLP weight: weight ratios. The resulting blends showed low viscosity, pH stability, good compatibility and good wetting behavior on all tested substrates. Adding AqFLPs to these emulsions can allow for better interaction with the substrate as well as the rubber due to its functionality and the hydrophobic polymeric backbone of the dispersions.
Adhesion of the AqFLPs and the blends to PET film was initially measured using a cross-hatch adhesion test. The coatings were tack free, showed good uniformity, good flexibility and no cracking upon bending. The results demonstrated that only blends containing AqFLP-3 and AqFLP-4 show excellent adhesion to the film as no apparent peeling of the dried coating was evident. Based on this data, AqFLP-4-based latex blends were then tested for adhesion between EPDM rubber and various substrates.
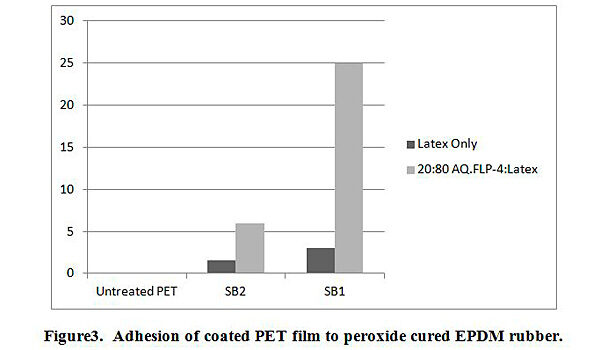
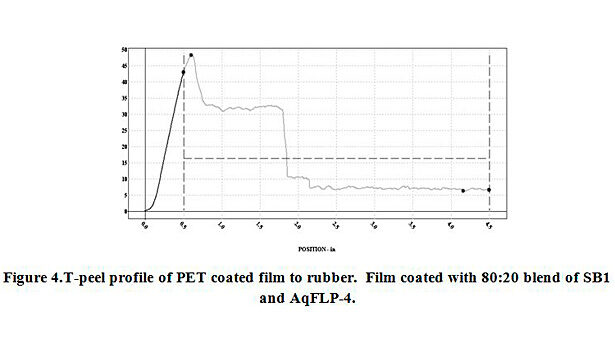
The additions of AqFLP-4 to the three emulsions showed substantial improvement in adhesion between coated PET film and EPDM rubber. The greatest improvement was demonstrated by the 80:20 ratio blend with SB1. Though the adhesion value (percent load) shown in Figure 3 is ~25 lb/in, the initial load average is 40 lb/in. Inspection of the test sample indicated the mechanism was clearly cohesive failure of the rubber. Therefore, the percent load value recorded does not reflect adhesive failure, but rather, is a function of the tear strength of the rubber. The mechanism is confirmed by inspection of the T-peel profile as shown in Figure 4. A slight improvement in adhesion is also observed with the blend using SB2.
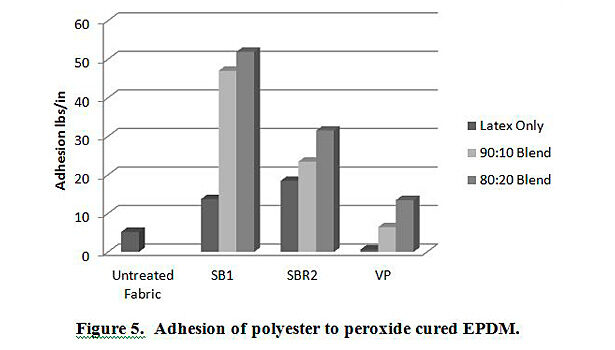
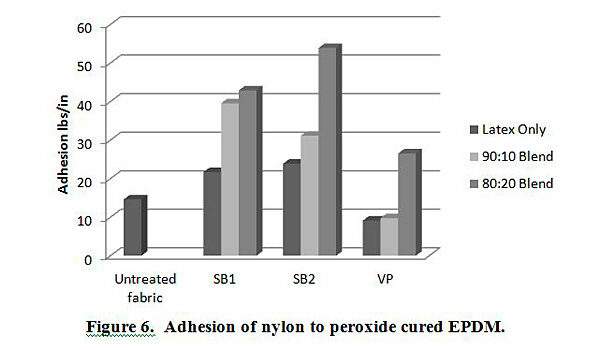
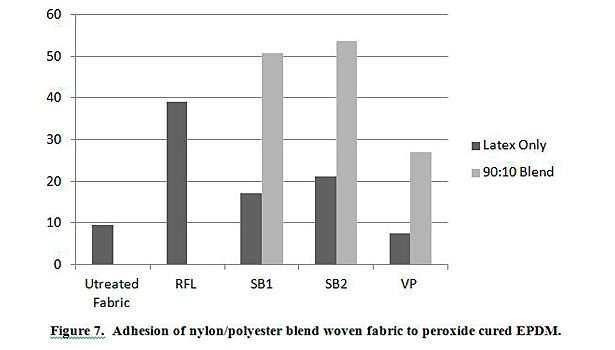
In addition to testing adhesion between coated PET film and EPDM peroxide-cured rubber, adhesion was also tested between EPDM and polyester, nylon and polyester/nylon fabric treated with AqFLP-4/latex blends. Figures 5 and 6 provide a comparison of the adhesion as a function of AqFLP-4/latex blends for both polyester and nylon fabrics, respectively. Though the greatest improvement with the addition of AqFLP-4 is demonstrated by blends using VP latex (96% increase for polyester and 63% increase in nylon), blends with VP latex show the lowest total adhesion values when compared to blends with SB1 and SB2. There is an overall increase in adhesion when increasing the amount of AqFLP-4 in the blend. All samples show better adhesion at the 80:20 ratio. Figure 7 shows an improvement in adhesion of the polyester/nylon blend fabric treated with a 90:10 ratio blend compared to RFL treated fabric.
In addition to T-peel testing, adhesion was also evaluated by rubber coverage on fabric. Figure 8 shows the rubber coverage difference between two strips of nylon and rubber where the nylon is coated with blends of SB2 with AqFLP-4 at two different ratios. The 80:20 ratio blend showed the most rubber coverage, which correlates with the higher adhesion values previously discussed.
The increased adhesion values for nylon compared to polyester may be explained by the increase in percent dip pickup measured for each treated fabric. The average pickup in polyester samples was 10% as opposed to 13% for nylon.
Conclusions
Aqueous dispersions of functional low-molecular-weight hydrocarbon polymers (AqFLPs) can be blended with SB and VP latexes as adhesion promoters for new textile treatment formulations. The AqFLPs can also be used to improve water and chemical resistance. Future work will include evaluating adhesion of the textile substrates to sulfur cure rubber as well as treating other substrates such as carbon fiber, aromatic polyamides (Kevlar), polyolefin-based nonwovens and metals.
In addition, AqFLPs may also be used as additives in other water-based formulations such as adhesives, coatings, paper sizing, construction materials and composites. The aqueous dispersions can also be used to pre-coat high-surface-energy fillers to improve wetting and ultimate dispersion in elastomers and thermoplastic/thermoset resins.
Acknowledgment
The author would like to thank Barry Evangelist for his work developing and performing the testing protocols used in the study. The author would also like to acknowledge Fabien Salort, Stephane Miallet, and Steve Henning for their contributions in developing this technology.
References
1 W.B. Wennekes, and J.W.M. Noordermeer, Rubber Chem. Technol. 80, 545 (2006).
2 S. Luo, W. J. Van Ooij, E. Mäder and K. Mai, Rubber Chem Technol. 73, 121 (2000).
3 A.S. Estrin, R.W. Nalepa, Rubber Division Meeting, ACS, Chicago, IL April 1999.
4 J. Austin, Polymers in Cables, Miami, FL March 2011.






Report Abusive Comment