
To understand the function of coalescing aids, it is necessary to examine the film formation process of a latex paint (see Figure 1). The emulsified polymer that will ultimately form the paint film exists as tiny droplets of solid material dispersed in water. On a porous substrate, some water and - depending on solubility - some coalescing aid will migrate into the substrate. As the amount of water decreases during the drying process, the latex particles are forced closer and closer together. When the repulsive forces on the droplet surface can no longer keep the particles separated, the emulsion collapses, and the system solidifies.

The ability of a polymer to form a film is a temperature-related phenomenon. Each polymer composition has a characteristic temperature below which it can no longer form a film. This is called the minimum film-formation temperature (MFFT) of the polymer. This property is normally measured using a MFFT bar apparatus according to ASTM Method D 2354. Polymers with low MFFT values form films easily, but the films have undesirable properties for paint applications such as low durability or high surface tack. The polymers generally used in paint formation have MFFT values in the range of 15-45˚C. These emulsions form durable, nontacky films when dried above the MFFT; however, in the hands of a painter, latex paint may have to be applied at temperatures approaching 0˚C. To form a good film at temperatures below the MFFT of the base emulsion, it is necessary to add a coalescing aid to the system.
A coalescing aid is often referred to as a temporary plasticizer for a film-forming polymer. The function of the coalescent is to soften the polymer during the crucial period of fusion so that the individual particles will combine to form a continuous film. The coalescing aid must then slowly evaporate, thereby allowing the paint film to develop its maximum durability and appearance properties. An ideal coalescing aid must fulfill three criteria. First, the coalescent must be an active solvent for the base polymer of the system. Second, the coalescing aid must efficiently lower the MFFT of the system. Finally, a coalescent must have an evaporation rate much lower than that of water. In addition to these three properties, it is highly desirable that the coalescing aid has very low solubility in water. The following discussion will demonstrate how Texanol Ester-Alcohol (E-A) meets all of these requirements and explain why it is the most widely used coalescent for latex paint in the world.
The first requirement for a coalescing aid is compatibility with the base polymer. Compatibility is determined by examining the solubility of the polymer in the material. The chemical name of Texanol E-A is 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate. The chemical structure is shown in Figure 2. Texanol E-A includes both hydroxyl and ester functional groups in its structure. These groups give Texanol a structural resemblance to most of the polymers used in paint formulation. This chemical similarity makes Texanol a strong active solvent for almost all of the acrylic, polyvinyl acetate, and polyvinyl chloride polymers commonly used to formulate paint. Texanol is also highly hydrophobic. This is very important because in the paint system, Texanol is generally absorbed directly into the emulsified polymer particles where it is most effective in reducing the effective MFFT of the system.
One can confirm the compatibility of Texanol and a particular resin by consulting the Resin Solubility Chart from Eastman.1 This publication gives solubility information for many common coatings resins in Texanol. The nearly universal compatibility of Texanol is one of the features that enhances its use. In most cases it is not necessary to confirm the solubility of a given polymer in Texanol. It is only necessary to determine the desired level of addition.
The second characteristic that differentiates coalescing aids is efficiency. Each polymer has a characteristic glass-transition (Tg) temperature, which defines the baseline MFFT. When a compatible coalescing aid is combined with polymer, the MFFT of the polymer-coalescent system decreases as the coalescent concentration increases. At some level of addition, the MFFT of the system will reach the desired level of performance for the formulated paint.
The efficiency of the coalescing aid is sensitive to the structure of the polymer. In other words, the quantity of a given coalescing aid needed to achieve the desired performance level will change for every polymer type. For example, it may take 3% by weight of the solids of the system for a given coalescent to produce an MFFT of 10˚C. Another coalescent might require a 6% addition level to achieve the same 10˚C MFFT with the same polymer.
The efficiency of the coalescing aid is an important determinant of the final cost of the paint. The cost/benefit ratio for a given coalescent must be determined for each formulation. In the preceding example, the coalescing aid requiring a 3% addition level could cost twice as much as the material with a 6% addition level and have a cost/benefit ratio equal to the less expensive material. When other important considerations such as odor, gloss, color development, or VOC level of the paint are taken into account, the benefits of a more expensive coalescent may outweigh those of a lower cost material. While Texanol is sometimes not the most efficient coalescent available for a given system, when the total cost/benefit ratio and other factors are considered, Texanol has proven to be the material of choice worldwide.
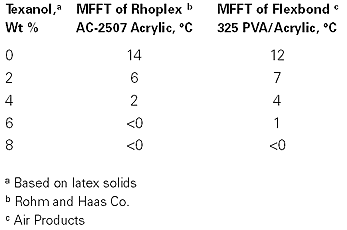
To give an idea of the concentration of Texanol required in a paint system, the data in the table (p. 70) gives the response of two commonly used emulsions to the addition of Texanol. These results show that the quantity of coalescing aid required to achieve the common industry standard of 5˚C minimum application temperature depends on the latex composition.
The third important characteristic of a coalescing aid is a low rate of evaporation. Pure water at 0% relative humidity has an evaporation rate of 0.48 at 25˚C on the n-butyl acetate scale (n-BuAc=1). At 80% RH, the evaporation rate of water drops to 0.09 compared to n-butyl acetate. An effective coalescing aid must have a rate of evaporation significantly slower than water in any drying condition. Furthermore, the formation of a paint film with maximum durability requires the coalescing aid to remain in the film on the order of 100 hours at an effective concentration. The evaporation rate of Texanol is 0.002 on the n-butyl acetate scale. This slow evaporation rate assures that the Texanol will remain in the drying paint film for sufficient time to assure proper film formation.
The evaporation rate of Texanol makes it ideal for most flat or semi-gloss paint applications. In high-gloss paint, the concentration of base polymer and consequently the quantity of coalescent required is higher. In high-gloss applications, the evaporation rate of Texanol is too slow, possibly causing slow drying and a tacky paint surface. When the volatility of Texanol is too low, Eastman EEH is often the preferred coalescing aid. In these demanding applications, Eastman EEH is superior for two reasons. First, Eastman EEH is generally 20-30% more efficient than Texanol so the relative concentration of coalescing aid is less. Second, the evaporation rate of Eastman EEH is 0.003 (n-BuAc =1) or 50% faster than Texanol.
The final desirable property for a coalescing aid is low solubility in water. While there are many materials that are quite satisfactory in the other three criteria, water solubility can be a very significant factor in the final performance of a given coalescent in a paint formulation. Water solubility determines how a coalescing aid is partitioned in the paint system. In other words, water solubility determines exactly where the coalescing aid is concentrated in the paint.
A water-insoluble coalescing aid mainly concentrates inside and on the surface of the emulsion particles. When the emulsion collapses during drying, the coalescing aid is concentrated at the point of greatest effectiveness-dissolving and softening the polymer.
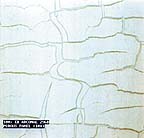
A water-soluble coalescent faces two problems. First, a water-soluble material tends to concentrate in the water phase of the emulsion. Figure 1 shows that when applied to a porous substrate, like most architectural construction materials, part of the coalescing aid migrates into the substrate and is not present for softening the polymer at the onset of coalescence. At some point, a water-soluble coalescing aid must combine with the polymer to be effective. Instead, water-soluble coalescing aids tend to be carried out of the system by the evaporating water due to azeotrope effects. Both of these problems can make a coalescing aid fail in actual paint application conditions when laboratory tests indicate good performance. Figures 3 and 4 show how a coalescent that works well in laboratory MFFT determinations can cause application problems because of its solubility in water.
In MFFT testing using the same latex, Eastman EB (ethylene glycol monobutyl ether) is actually more efficient at reaching an MFFT of 4.5˚C than Texanol. In these samples, however, it is apparent that Eastman EB added at a level of 6 phr (6% based on the dry weight of the polymer) does not form an acceptable film at 4.5˚C when coated on a porous panel. The reason for this is that the complete solubility of Eastman EB in water makes a large difference in actual application performance even though testing on an MFFT Bar with a metal surface indicates that Eastman EB is more efficient than Texanol. On the other hand, the panel shown in Figure 4 containing the same 6 phr level of Texanol forms a completely continuous film of paint even in the difficult drying conditions of 4.5˚C and 95% relative humidity. Once again, the superior cost/ benefit ratio of Texanol wins out over a lower cost but less effective coalescing aid in an actual application.
It is also important to consider that coalescence is a process that occurs over several days. Paint applied at a daytime temperature of 10˚C can easily be exposed to nighttime temperatures of 5˚C - when the most active coalescence of the film is occurring.

In lab drying conditions, the gloss of the series of samples was almost equal. The results for 60˚ gloss ranged from a high of 73 for Texanol and Eastman EEH to 70 for Eastman EB to 65 for Dowanol DPnB. In the more severe low-temperature/high-humidity conditions, the results were much different. The sample produced with Texanol has a 60˚ gloss reading of 68 compared to 64 for Eastman EEH. The sample made with Eastman DB showed a 40 value and the paint film was grainy. The sample with Dowanol DPnB could not be tested because the paint gelled at 4.5˚ and could not be applied.
The scrub resistance test closely followed the 60˚ gloss results. The four paint samples were applied to test panels and allowed to dry for 48 hours at the two environmental conditions. The eight panels were then aged for six weeks at standard lab conditions and then tested for scrub resistance using ASTM method D 2486. At normal lab conditions, the paint sample using Eastman DB was used as the control and had a resistance of 675 scrubs. Texanol resisted 1,350 scrubs or 200% of the control value. Eastman EEH lasted for 1,400 cycles (207%), and Dowanol DPnB held for 750 scrubs (111%). At the low-temperature/high-humidity conditions, the control using Eastman DB produced a grainy film but still developed scrub resistance of 570 cycles. Texanol produced a scrub resistance of 805 (141%), while Eastman EEH fell to 400 (70%). Once again, the sample using Dowanol DPnB gelled at 4.5˚C and could not be tested.
This study demonstrates that Texanol produced excellent results for 60˚gloss and scrub resistance even when applied in the most demanding conditions. It outperformed the more efficient Eastman EEH and the lower-cost Eastman DB in most of the tests. Although this study was limited to one paint formulation, it demonstrates again why Texanol is the preferred coalescing aid for most types of paint worldwide.
Another way Texanol improves the performance of paint systems is in the development of color. The coalescing aid in the system affects the surface roughness of the paint film. This in turn can change the reflectance of light from the film's surface and therefore the color of the dried paint film. The data shown in Figure 6 illustrates the effect of the coalescing aid on color development.
This test was made using flat latex paint samples tinted with a blue pigment and dried at 6˚C for 24 hours. The color of the samples was then measured with a Hunter D25D2 Color Difference Meter using the blue scale. The higher the number on the scale, the more intense the blue color. It is clear that the addition of Texanol noticeably improves the color development of the system. This effect obviously can have a major impact on color matching and the pigment concentrations needed to achieve a given color. Texanol has proven in many evaluations to provide superior and consistent color development over a range of application conditions compared to other coalescing aids.





Report Abusive Comment