Methyl methacrylate (MMA) is an essential monomer for many acrylic-based polymers, and its extensive range of applications include sealants, adhesives, exterior paints and coatings, and shatter-resistant panels for windows and bathtubs. The primary use of MMA comes from its role as a monomer in the polymerization of polymethyl methacrylate (PMMA), a highly transparent and durable polymer. One of the most prevalent uses for PMMA occurs in the manufacturing of paints and coatings. PMMA plays an essential function in the production of automotive paints, inks, metal coatings, industrial coatings, floor polishes and much more.
PMMA is synthesizable in solution, emulsion or bulk polymerization. Real-time characterization for such a process is preferable to the traditional QA/QC procedures, which require an offline lab analysis of crucial polymer properties.
ACOMP is a real-time, online monitoring system that continuously analyzes polymer properties during production. The smart manufacturing system uses a well-known, nonchromatographic technique to measure monomer and polymer concentration, intrinsic viscosity (IV) and weight-average molecular weight (Mw). The system’s automation and analysis software handles data acquisition, analysis and reporting. In addition to an array of detectors, the system includes multiple prescriptive maintenance sensors, qualifying it as self-aware. For industrial or pilot-scale applications, the system is available in a fully purged, Class I, Division II cabinet.
Fluence Analytics, the manufacturer of ACOMP, recently released a third generation of the system. The latest generation can process polymer at a higher temperature flow through detectors, leading to better-quality data for high-molecular-weight polymers. Other enhancements include broader analytics functionality, including optional machine learning, an improved user interface, an upgraded detector train, an industrial-grade pumping system with more diagnostics and system health monitoring capabilities, and new smart sensors.
The real-time insight generated by ACOMP enables polymer manufacturers to immediately respond to process anomalies, adhere to tighter polymer quality control specifications, and optimize polymer production for continuous, batch and semibatch runs. This article describes how ACOMP was utilized to successfully monitor and characterize the free radical solution polymerization of MMA.
Monitoring the Synthesis of Polymethyl Methacrylate
The ACOMP method, in principle, requires the continuous extraction of a small sample stream of reactor contents that are then diluted and conditioned for detection by appropriately selected concentration and molar mass sensitive detectors. In this application of monitoring the methyl methacrylate polymerization, the ACOMP system uses an ultraviolet absorption detector (UV) to measure the concentration of monomer throughout the polymerization. The conversion of this monomer into polymer directly corresponds to the reduction of the UV absorption signal. This continuously monitored signal provides us with both fractional polymer conversion as well as polymer concentration. These results can also be used to determine the true polymerization kinetics and reactivity ratios of the monomer. To determine the molar mass properties of the polymer, both a single capillary dilute solution viscometer (DSV) and multi-angle static light scattering detector (MALS) are used to characterize the intrinsic viscosity and weight-average molecular weight respectively. In this experiment, the ACOMP system extracted a continuous sample from the reactor at a rate of 0.25 mL/min. This sample was then automatically and continuously diluted with butyl acetate to achieve a 55x dilution to the detectors. The objective of extracting and diluting the polymer sample prior to detection is to ensure that there are very little, if any, intermolecular interactions taking place that can influence the characterization of intrinsic polymer characteristics.
Reaction Methodology
In monitoring and characterizing the batch methyl methacrylate polymerization, the reactor was charged with 30% by mass of total reactant in butyl acetate, then initiated with 3.5% azobisisobutyronitrile at 65 °C. The reaction was carried out with a low-volume purge of nitrogen at 50 sccm to ensure that no oxygen was present to quench the free radical polymerization. All materials were sourced through Sigma Aldrich.
Figure 1 shows the normalized raw data from the MMA reaction. In following the x-axis of the graph, which represents the time scale, the beginning of the polymerization monitoring displays only pure solvent in the detector train. As there is neither monomer nor polymer present at this time, accordingly, there is no detector response. Once monomer is introduced into the detector stream, the UV absorption signal increases as the double carbon bonds of the MMA monomer absorb UV light. Note, however, that there is not yet a response from the DSV and MALS detectors, which are molar mass sensitive. At 4,000 sec, the initiator is added to the reactor and the detector signals begin to change. The UV absorption signal starts to drop as the double carbon bonds of the MMA monomer are converted into the polymethyl methacrylate polymer chain. Additionally, the DSV and MALS detector signals begin to increase as the polymer chains increase in size and concentration. This real-time and continuous raw data is then used to characterize the key reaction processes and polymer properties.
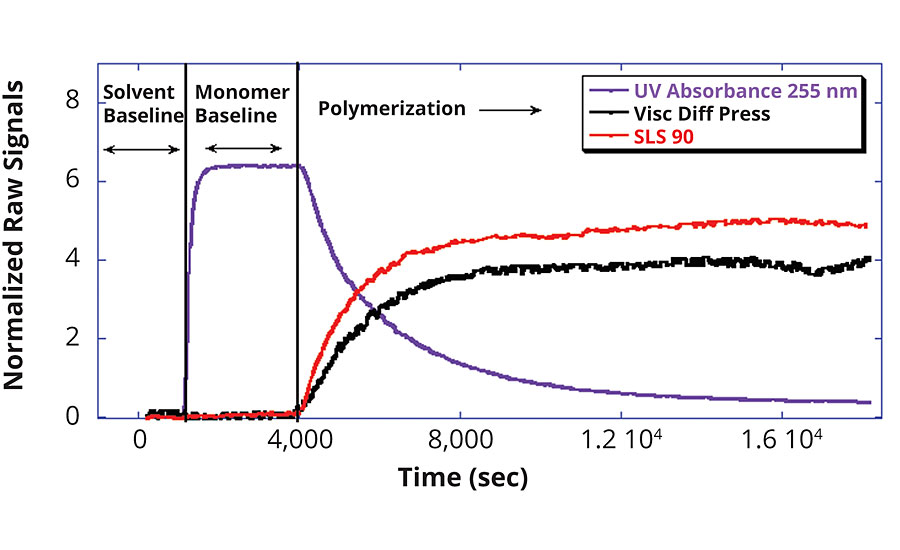
Real-Time Polymer Properties
The concentrations of monomer and polymer as displayed in Figure 2 are determined using the UV detector. Since this is a batch reaction, the polymer concentration increase is directly proportional to the decrease in monomer concentration.
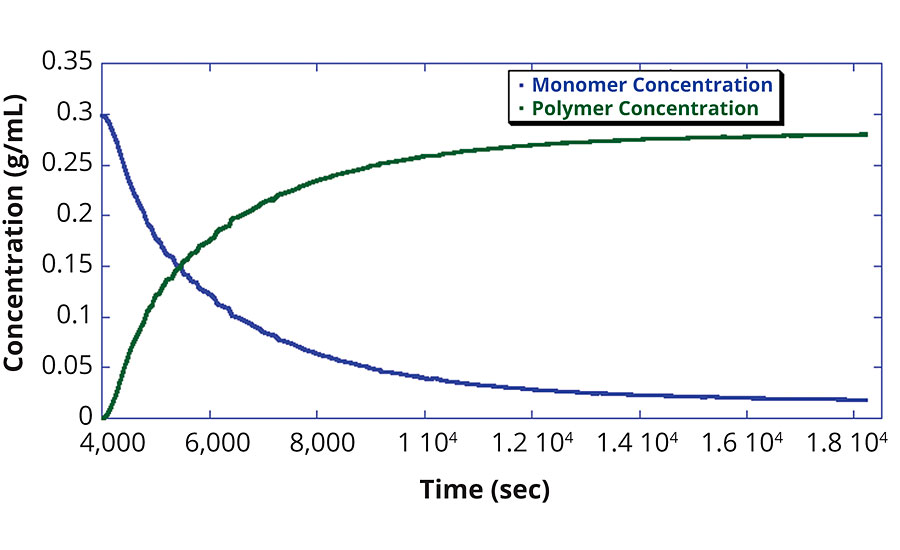
From the concentration data, the fractional polymer conversion is determined, as exhibited in Figure 3. Conversely, Figure 4 shows how the monomer concentration of this reaction was fitted with an exponential to determine the rate of monomer consumption. This exponential fit can then be used to determine when a target residual monomer concentration will be achieved. This determination is often necessary to ensure that harmful monomers are sufficiently polymerized to yield the product safe for end-user handling and processing.
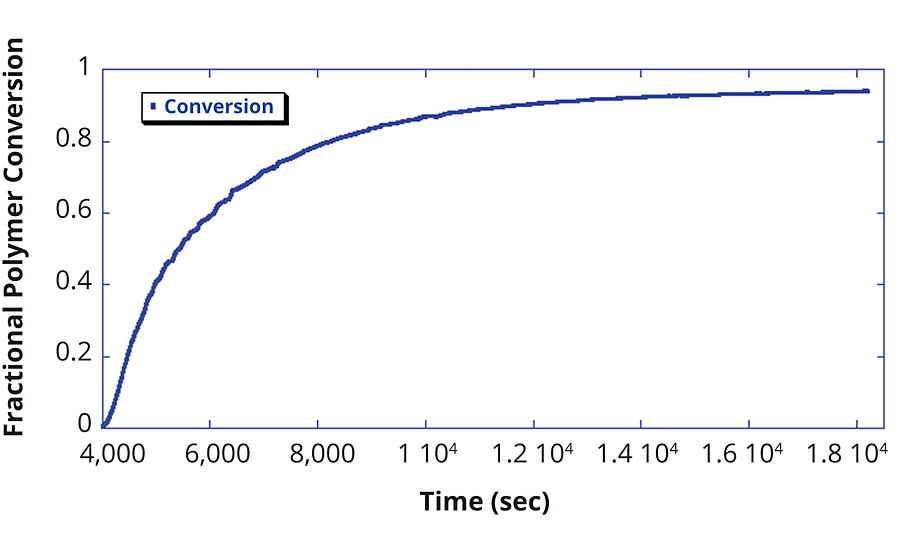
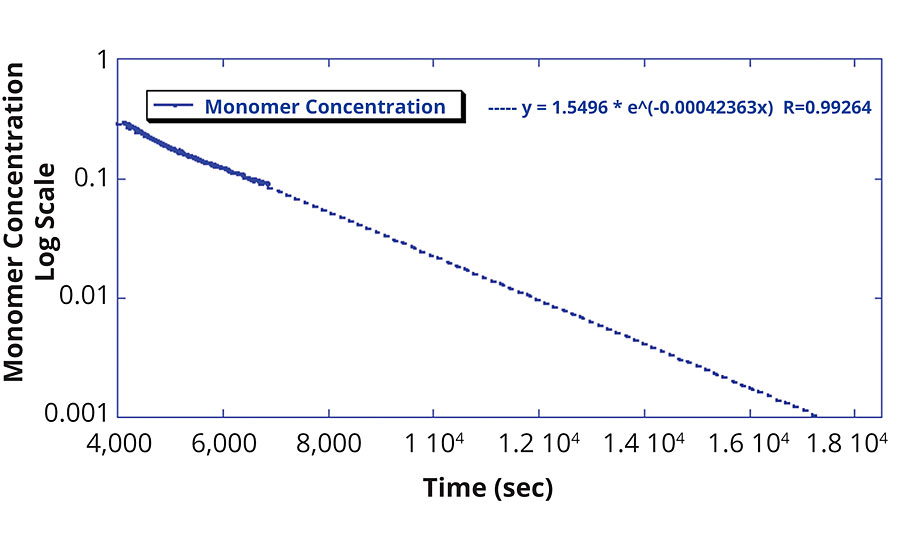
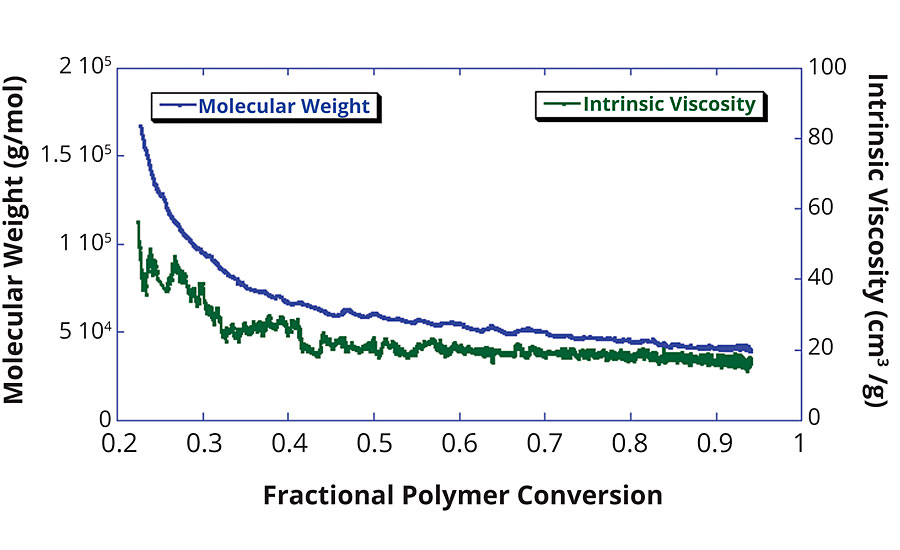
Figure 5 shows the evolution of the polymer’s intrinsic viscosity and weight-average Mw throughout the polymerization. This plot illustrates a classical evolution of Mw and IV for a free radical polymerization in which the polymer chain length is very high at the beginning of the polymerization when there is an abundance of monomer present. As the polymerization proceeds and the concentration of monomer is continuously diminished, the growth potential for the polymer chains is limited. This leads to smaller and smaller polymer chains throughout the polymerization. The result of this is an increasing polydispersity and overall reduction in the weight-average molecular weight and intrinsic viscosity of the polymer end product. This effect, however, can be mitigated by either quenching the reaction when a desired Mw or IV is achieved, or by modifying the reaction procedure to a semibatch process in which monomer is fed into the reactor during the polymerization. Again, with the use of ACOMP’s online continuous monitoring capability and the continuous molecular weight feedback control (discussed in References 2 and 3), the desired Mw and IV can be purposely achieved rather than an anticipated outcome of the reaction recipe.
Value of Real-Time Data in MMA Polymerization
This application shows how ACOMP easily monitors the free radical solution polymerization of methyl methacrylate. This functionality allows researchers and polymer manufacturers to effortlessly monitor and characterize key polymer properties as the product is being produced, rather than having to perform post polymerization analysis with little insight of the evolution and kinetics of mass growth and monomer consumption. This provides for real-time insight into the causes and effects of recipe and reaction condition changes such as monomer, initiator and temperature. Additionally, with the ability to monitor and control semibatch polymerization reactions, purpose-driven and specialized polymer properties can be achieved through the real-time monitoring and control of molecular weight and intrinsic viscosity.

References
1. Automatic Continuous Online Monitoring of Polymerization Reactions (ACOMP) and related techniques: Encyclopedia of Analytical Chemistry, Wiley 2013.
2. Automatic Control of Polymer Molecular Weight During Synthesis: Macromolecules, 2016, 49 (19), pp 7170-7183.
3. Automatic, Simultaneous Control of Polymer Composition and Molecular Weight During Free Radical Copolymer Synthesis: Polymer 136, 2018, pp 235 - 247.
For more information, contact info@fluenceanalytics.com.







Report Abusive Comment