Some steel structures, dating back thousands of years, are still in good condition, without losing metal layer, luster, or their aesthetic look. The technique of making superior-quality steel and protecting the metal has obviously been available for years.
Today however, due to economic aspects, a variety of steel is used in many application areas from pipelines to bridges, transportation materials, marine, etc. However, due to severe corrosive conditions, these metal parts are subjected to many critical exposures such as humidity, salt spray, acid/alkaline chemical fumes, mechanical damage and atmospheric changes. The environment consists of a variety of materials, which initiate and enhance corrosion. Severe conditions further aggravate and result in damage. The following discussion covers corrosion theory and different techniques available today to fight against corrosion.
Corrosion Theory and Concepts
Various metals, such as steel, are formed by special high-temperature treatment. However, these metals are unstable in ordinary aqueous environments. The metal gets affected quickly, when exposed to water, humidity, salt water, chemicals and chemical fumes, and then combines chemically to form compounds with these metals, finally resulting in rusting, corrosion or a tarnishing effect.
The process of corrosion starts whenever a metal surface is exposed. The chemical reaction is accompanied by the passage of electrical current. It is an electrochemical process. The process of corrosion is known as an anodic reaction process, whereby metal-dissolving ions are generated. The following factors are primarily responsible and have influence on the process of corrosion.
- atmospheric conditions, environmental cycle;
- too much humidity, salty conditions;
- atmospheric chemical fumes;
- oxygen availability;
- composition and quality of the metal;
- storage conditions, pretreatments;
- chloride ion penetration and contact; and
- sulfate content and conversion to sulphide.
Types of Corrosion
The first step is establishing the anodic or cathodic site. The process occurring at the anodic site is the dissolution of metal as metallic ions, and converting these ions into insoluble corrosion products, such as rust. At this stage a process of metal destruction starts.
Pitting Corrosion
The initiation of pitting occurs when an electrochemical or chemical breakdown exposes a small local site on the metal surface to a damaging species such as chloride ions. The pit is the anode of an electrochemical corrosion cell, and the cathode of the cell is the non-pitted metal surface.
Crevice Corrosion
A portion of a metal surface is shielded in such a way that the shielded portion has limited access to the surrounding environment.
Stress Corrosion Cracking
Localized corrosion, which produces cracks in metals, stresses the breakdown of the passive layer on the metal surface.
Inter-Granular Corrosion
Formation of small crystal grains, thereby weakening the metal surface.
Uniform Corrosion
Corrosion spread uniformly over all the surface.
Galvanic Corrosion
Corrosion responsible when two different metal surfaces are in contact or used in composition.
Erosion Corrosion
Erosion corrosion results from the disruption of the protective passive films. The rate of corrosion is higher and faster on an uninhibited surface and slower on an inhibited surface due to the prevention of contact with air, oxygen and other elements.
Choices for Corrosion Prevention
In the olden days choices for preventing corrosion were very limited. Today, due to technology developments and upgrades, a number of solutions are applied in practice. Following are the four major solutions that are widely used.
- Composition of the metal. In the steel, further modifications are done by incorporating various other non corrosive metals – SS304, SS316 are some examples. This, however, is a very costly proposal, but may need to be used depending on the performance expectation.
- Plastics or polymers are fused, deposited or fixed on the metal; for example various pipes, instruments, machinery components, etc. are prevented from corrosion in this manner.
- Applications of a sacrificial coat/deposit, like zinc galvanization, or tin coated; the process is very costly.
- Barrier coatings – Most widely accepted and used technique, whereby a polymer or anti-corrosive coating is applied on a metal surface of any shape or size to cut off the corrosion-leading elements, thus forming a barrier coat.
Range of Anti-Corrosive Coatings
In the past, alkyd-based red oxide primers, zinc chromate primers and micacious oxide-based coatings were in common use for corrosion prevention. Now, conventional high-solids two-component epoxy paint or solvent-free epoxy paints are used. Even for high-performance needs glass flake epoxy coating is used. Epoxy zinc silicate is today a most acceptable product. Sometimes polyurethanes and polyureas are applied for specific jobs. However all these systems require too much surface preparation, primer, intermediate, and topcoat applications. The composite thickness is very high, and it takes a lot of time, labor, skill etc. The latest remedial solution that is popular, specifically in the United States, are anti-corrosive coatings based on gelled calcium sulfonates. The unique features of these coatings are:
- minimal or no surface preparation is required;
- a single coat, without any primer, intermediate application;
- film thickness can be applied from 40-400 microns in a single coat;
- weldability;
- resists undercutting; and
- very high corrosion resistance.
A comparison of an epoxy mastic-coated panel, 500 microns DFT, and an alkyd modified with gelled calcium sulfonate inhibitor, 260 microns DFT, was conducted for 50 months exposure time at a New Jersey (U.S. east coast site). The epoxy coating failed after 50 months exposure, and there was no effect on the panel based on calcium sulfonate-modified alkyd film.
Gelled Calcium Sulfonate Chemistry Features
Gelled Calcium Sulfonates
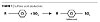
|
| Figure 1 Click to enlarge |
Thixotropic over-based gelled calcium sulfonates are produced via a complex series of chemical reactions. Some of the important steps of the manufacturing process are shown in detail in Figures 1-4.
Sulfonation
Sulfonic acid is first formed by reacting an alkyd benzene compound with sulfur trioxide as shown in Figure 1.
Neutralization and Over-Basing

|
| Figure 2 Click to enlarge |
The sulfonic acid is then converted into the sulfonate-carbonate complex by neutralizing the sulfonic acid and reacting the metal salt with an excess of calcium hydroxide and calcium oxide to form the over-based complex. The carbonate formed in this reaction is amorphous in structure (Figure 2)
Carbonate Modification
The modification step converts the small amorphous carbonate particle structure (9 nm average) to a large particle size platy calcite crystal (200-250 nm average). This calcite structure, shown in Figure 3, imparts thixotropic viscosity characteristics to all products.
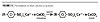
|
| Figure 3 Click to enlarge |
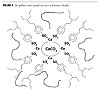
Sulfonate Complex
The calcium carbonate particles are tied to the sulfonate complex and will not settle out in a coating formulation (Figure 4). Competitive materials are often made by dispersing dry calcium carbonate, very fine mesh powder, into a sulfonate base, for cost reduction. This externally added carbonate is not chemically bonded to the sulfonate backbone and can thus settle down from suspension.
|
| Figure 4 Click to enlarge |
Various Functionality of Products
The products contain the thixotropic over-based calcium sulfonate-calcium carbonate complex dispersed in mineral spirits or in oil. These materials exhibit a variety of functionalities, which relate to the chemical structure of over-based gelled calcium sulfonate: hydrophobicity, polarity and corrosion inhibition. This is illustrated by the structure shown in Figure 5.
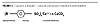
|
| Figure 5 Click to enlarge |
Hydrophobicity
Alkyl groups attached to the aromatic ring face away from the substrate and repel the ingress of moisture. These mostly saturated aliphatic chains are completely nonpolar, conferring on the complex the duality of being polar on one end and nonpolar on the other side. They also make hydrophobic compounds like waxes, oxidative petrolatums, oils and asphalts compatible in Jalpon coating formulations.
Polarity
The aromatic ring and attached SO3 group form a highly polar entity, which results in coatings with superb metal wetting and moisture displacement properties. The polar end of the sulfonate/carbonate complex attaches to metallic substrates and demonstrates exceptional adhesion even when the coating film is damaged. Very thin, less than 1 mil-based coatings, can offer up to 1,000 hours of protection in the ASTM B 117 Salt Fog Test. The moisture displacement property allows the application of coatings in less than optimal conditions.
Corrosion Inhibition
The calcium carbonate part of the complex functions primarily as an alkalinity reserve. The carbonate is slightly soluble in water and acts as a corrosion inhibitor by buffering the pH at the metal surface. A pH of approximately 10.0 can be maintained at the coating/metal interface, which delays the initiation of the corrosion process.
Anti-Corrosive Barrier Coatings

|
| Table 1 Click to enlarge |
The size and shape of the calcium carbonate particle is controlled during synthesis to form the platelet structure. These flat platelets form a barrier to air, moisture, and carbon dioxide to reach the metal surface, thereby stopping the electrochemical reaction process, and thus preventing corrosion. Thixotropic gelled calcium sulfonates are superior than commonly available calcium sulfonates. The features and benefits of gelled calcium sulfonates are shown in Table 1.
Performance in the United States
For the rehabilitation of the high-level bridges, the Federal Highway Administration (FHWA) has studied 2000 bridges out of 200,000 total bridges in the United States whose condition is critical. They have sought the help of polymer science and various laboratories including Turner-Fairbank Highway Research Centre (TFHRC), Powertech laboratories.
Testing was conducted by Powertech Labs Inc., of Surrey, British Columbia, where 16 coating systems were tested. Included were many multi-coat systems, some of which were single-component. A wide range of lab testing criteria were applied to each coating.
Of particular interest are durability tests conducted over aged alkyd substrates as well as blasted steel. One of the more interesting tests was an environmental/weathering test, “Envirotest” developed by KTA Tator, of Pittsburgh, PA.
This test includes immersion in salt water, exposure to heat and humidity and an ultraviolet light exposure. The test cycle consisted of exposure at 60 °C, used a 4% salt solution and exposure to UVB lamps. The chamber provides alternating exposure to the various environments by cycling 420° every 2 hours.
A calcium sulfonate (alkyd) system was ranked first in two of the four durability tests, including Envirotest on blasted steel, (SSPC-SP6) with test duration of more than 4,400 hours.
Conclusion
Anti-corrosive coatings based on gelled calcium sulfonates are new proven systems that are now available and used in the United States and other developed countries with a number of added advantages such as ease of application accompanied by high corrosion resistance.
Dr. Ninad Kondekar has worked for 38 years with various companies, including Asian Paints India; Pidilite Industries; National Paints, UAE; and Apcotex India.




Report Abusive Comment