When a luminescent pigment molecule is excited by light energy, they are excited to higher Singlet energy states, which then revert to their original ground state by emitting a portion of their absorbed energy. If decay is directly from S2 to S0 then fluorescence occurs; if the energy is dissipated by way of the triplet state (T1 or 2), then phosphorescence occurs.

Fluorescent Colorants
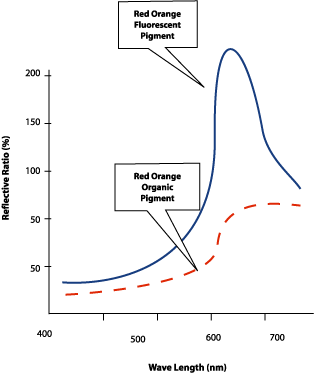
Fluorescence is a phenomenon that occurs when a substance absorbs radiation of a certain wavelength, or group of wavelengths, and re-emits photons of a different wavelength (see Figures 1-2).When certain compounds absorb light, an electron is excited to a higher vibrational energy state. The molecule then loses its excess of vibrational energy by collisions or by emitting a photon in the infrared or microwave bands and falls to the lowest vibrational level of the energy state. In addition, almost all molecules occupying an energy state higher than the second undergo internal conversion and pass from the lowest vibrational level of the upper state to a higher vibrational level of a lower state that has the same energy. From there, the molecules again lose energy until the lowest energy level of the first excited state is reached. From this level, the molecule can return to any of the vibrational levels of the ground state, emitting its energy in the form of fluorescence. Typically, molecules that display fluorescence are of two types -- organic compounds with a high degree of conjugated unsaturation and extended p- cloud structure, or inorganic compounds where it is relatively easy to promote an electron to a higher vacant energy level (usually a d- or f- level), and the molecule may be excited to a higher vibrational and rotational energy state. The spectrum of the emitted light is called the emission spectrum. Due to the conservation of energy, the emitted light is almost always at a higher wavelength.

The reflectance of an object determines the color. In a non-fluorescent molecule, photons are still absorbed but they immediately drop back to the original energy state, dissipating the energy in the form of heat or simply emitting a photon of equal wavelength. The result is a typical absorption spectrum, since absorption is merely the log (1/R). If there are molecules in the colorant mixture that fluoresce, the resulting spectrum is a combination of a number of factors, including the reflectance of the non-fluorescing materials, the absorbance of the fluorescent material due to the excitation of electrons to a higher energy state, and the emission spectrum of the fluorescing material. Truly a mess to interpret since both the absorption and fluorescence depend on the spectral power distribution in the illuminant used.


Daylight Fluorescent Pigments
Daylight fluorescent pigments are an American invention. In the 1930s, Bob and Joe Switzer experimented with ways of combining certain dyes and resins that produced colors far brighter than normal and that had the unique effect of "glowing" under UV or black light.Initially, these new colors and effects found their way into magic shows, stage shows and movie promotion posters. In 1936, they became associated with a firm in Cleveland, which produced the movie posters and moved to Cleveland from California.
During World War II, their focus was diverted to the manufacture of bright signal panels as used by the army. After the war, this technology was used in outdoor billboards where the message "jumped off the boards."
Fluorescent pigments are solid dye solutions in polar resin carriers. Fluorescent dyes in their undissolved state do not fluoresce. Once these dyes are dissolved in a thermoplastic or thermoset carrier resin and are ground into a fine powder, they become fluorescent pigments. Only a few fluorescent dyes that are commercially available can be made into pigments. Some of the most important dyes used are based on Rhodamine B (invented in 1877). The most common fluorescent dyes used are shown in Table 1.

- Thermoplastic Pigment (Melamine Formaldehyde Type) One of the oldest types of carrier resins is based on para toluene sulfonamide (PTSA) --melamine -- formaldehyde, which is fully condensed resins and melt at a range of about 115-135 deg C, are clear, brittle and easily pulverized into a pigmentary form. (Used in printing inks.)
- Thermoset Pigment (Melamine Formaldehyde Type)
These types were the next to be developed. Through post curing and some chemical modification of the PTSA-MF resins solvent resistance was increased and softening temperature was raised to 175 deg C. (Used in printing inks.) - Thermoset Pigment (Benzoguanamine Formaldehyde Type) A newer type, where the melamine component of the PTSA-MF is replaced by benzoguanamine. This results in a spherical particle size rather than ragged edges. (Used in printing inks.)
- Thermoset Pigment (Benzoguanamine Formaldehyde Type)
These are specifically developed for plastics to minimize the plating tendency of all other types. They contain only benzoguanamine formaldehyde type matrix. (May be used in printing inks.) - Thermoplastic Pigment (Polyamide Type) These types were developed for use in plastics. They are based on aliphatic diamines and isophthalic acid with melting points from 110-150 deg C, depending on the intended application.
- Thermoplastic Pigment (Polyester Type) These also were developed for use in plastics. They are based on polyfunctional glycols and phthalic anhydride. They have lower softening points (around 90 deg C) and are considered less heat stable than PA types. They do not contain any formaldehyde.
- Thermoplastic Pigment (Vinyl or Acrylic Type) These are mainly used for calendered vinyl and the matrix is based on PVC or an acrylic resin. They do not contain any formaldehyde and display very high color strength.
- Aqueous Dispersions (Acrylic Type) These are a newer development in fluorescent pigment manufacture and are specifically designed for printing and writing inks. They are manufactured by adding fluorescent dyes into acrylic monomer and then polymerizing, thus creating colored latex particles (globules). They are formaldehyde free and have a very fine particle size (sub micron), which is very suitable for ink manufacture. (Used in printing inks)

UV Fluorescent Pigments
Inorganic Fluorescent Pigments (IFP) are further distinguished from daylight organic fluorescent pigments by the fact that most are either colorless or have very pastel colors under daylight, but when excited by UV radiation, they fluoresce in relatively bright colors. The excitation energy can either be short or long UV. The visible color of these inorganic pigments is due entirely to emitted radiation and they are additive colors. Thus, a blend of equal parts of a green glowing and a red glowing pigment results in a yellow glowing pigment.These chemicals encompass a variety of luminescent chemicals such as lamp, TV and CRT phosphors, but this article will cover only ones that are used in "coloration" of inks, coatings, plastics, and similar applications.
With organic compounds, luminescence is essentially based on localized electronic systems within the organic molecules. With inorganic compounds, however, the luminescence is determined by their lattice structures and thus their luminescence is altered or disappears completely when their crystal structure is altered in any way (melting, decomposing or breaking) (see Figures 4a-4b).4
The classical IFPs have been based on the zinc sulfide and zinc cadmium sulfide. These inorganic fluorescent pigments (e.g. [Zn, Cd]S doped (activated) with silver, copper, or manganese) are today considered "toxic" (because of their cadmium content). The activator type and amount used determines the color of the fluorescence. For example, when copper is used as the activator the color can be varied from green to deep red, whereas when silver is used, the fluorescence color ranges from deep blue to deep red.
Therefore, a special range of inorganic luminescent pigments (some of them based on rare earth elements and some having quite complex crystal structures) that has been specially surface treated for ease of printing, has been developed to satisfy the printing industry's needs. These pigments have found use, especially in the security printing industry, and they have been designed with this specific industry's needs in mind.
These pigments are composed of a microcrystalline host (or matrix) based on rare earths and an activator, i.e., a small amount of intentionally added impurity atoms distributed in the host crystal. Some of such matrices are: oxides, sulfides and oxysulfides of rare earth elements, and aluminates of alkali earth metals.
An important use of these pigments is in document security applications. One has to take into consideration the security printing industry's stringent requirements for the pigments that it uses. In addition to being as "forgery proof" as possible, the end product should also pass other quality and durability requirements. These include the following.
- The pigment should possess excellent lightfastness.
- It must have superior rub (dry & wet) fastness.
- Its fastness to various solvents and chemicals must be excellent, i.e., ethyl acetate, ethanol, acetone, trichloroethylene, benzene, xylene, bleaching solutions, caustic soda, acetic acid, hydrochloric acid, perspiration, soap, detergent, etc.
- It must have a particle size small enough to be printable with all printing processes.

Users of UV fluorescent pigments must be aware of their shortcomings. These can be summarized as follows.
- Particle size: Average particle size is larger than organic pigments, i.e., sub-micron vs. larger than 2-4 microns. They should also not be ground too fine as their afterglow is dependent on their crystal structure. Once this is broken, they do not glow.
- Abrasiveness: As they are based on inorganic molecules (some are even based on aluminates) they can be quite abrasive. Therefore, users should be careful with damage on gravure rolls and offset plates.
- Color Strength: As their color formation is dependent solely on the fluorescent component, more than normal quantities might be needed in order to be able to get bright enough colors.
- Additive Color Concept: Remember that these colors are additive, a mixture of red, blue and green results in a white, and not a black. Therefore, if prints must be true to their intended colors, different filters should be used.


Optical Brightening Agents (OBA)
Optical brightening agents (OBA), or fluorescent brightening agents (FWA), are a particular class of organic compounds that have very specific fluorescent properties. They have excitation maxima in the very near UV (340-400 nm) and emit in the 430-460 nm wavelength ranges.5 The major uses of these products are for hiding the yellowish tint of paper, textiles, coatings and plastics by giving them a bluish tint. Typically their chemistry is based on the stilbene molecule and is modified to enhance solubility and uptake depending on the substrate in which they are used.Another use for these products is printing invisible markings in security applications, similar to UV fluorescent pigments. (Most probably the sample in Figure 6 has been manufactured using an OBA.)

IR Fluorescent (Anti-Stokes) Pigments
Another class of specialty pigments that is not known much in industry is the IR fluorescent or anti-stokes pigments. The Stokes Law states that when a molecule is excited by a source of certain wavelength, the resulting color output is of a higher wavelength. Examples are excitation by UV light that results in various visible colors that are of longer wavelengths. But IR fluorescent pigments do not follow this law, they are excited by IR energy, and either emit in the visible region or in the IR region but at a shorter wavelength.These products can also be used in printing inks by taking the same precautions as in IFPs. In order to detect them special near IR lasers or LEDs are needed.

Phosphorescent Pigments
Phosphorescence is similar to fluorescence in as much as it is a phenomenon that occurs when a substance absorbs radiation of a certain wavelength, or group of wavelengths, and re-emits photons of a different wavelength. The difference is in the emission time. As a rule of thumb, if emission stops after the excitation source has been removed, then the resulting luminance is called fluorescence; if emission continues (so called "afterglow") then it is called phosphorescence. There are also phosphorescent minerals, but their phosphorescent intensity is very low and actually beyond the capabilities of the human eye to detect. The first recorded observation of inorganic luminescence by V. Cascariolo ca. 1603 was also phosphorescence.6 One way of observing this very low phosphorescence is to place them in front of a photographic film, after being activated by a suitable light source, and record the image developed on the film.
Later on, through improvements in their manufacturing process, zinc sulfide:copper doped (ZnS:Cu) type phosphorescent pigments acquired afterglow characteristics that were strong enough to be usable in safety signage. These pigments had a green afterglow with a peak at 520 nm (see Figure 7).
These pigments were (and are still) also used in toys, novelties, and textile printing. However, their main deficiencies were that:
- Their particle size was too large for printing except silk screen (average 25 mm);
- To obtain decent afterglow, a very thick layer containing a very large amount of pigment was required;
- Afterglow is particle size dependant, larger particles have brighter afterglow.
The invention and further development of this new class of pigments opened new possibilities in a multitude of ways, because an acceptable level of afterglow was possible by using less pigment and the afterglow time had increased tremendously.
These AEA pigments were also manufactured in a blue green (aqua) and violet afterglow colors. Figure 8 shows the excitation and emission curves of ZnS:Cu and green and blue green emission AEA pigments. These newly invented pigments initially had their own problems, mainly:
- They had large particle sizes: >20 microns(developed for the plastics industry);
- They were hard and abrasive (6.8-7.0 on the Mho scale);
- They lost their after glowing capability when moist (while in powder form).


Optically Variable Pigments
Optically Variable Pigments (OVP[R])8 show angle-dependent color and lightness effects which are based on reflection, interference and absorption phenomena of visible light in pigmentary multilayer systems.9OPVs' color changing ability is based on a physical phenomenon known as the Fabry P?rot interference principle, which was first observed in 1897 (this method was originally used for determining the wavelength of light).
OVPs are multi-layer films of semi-transparent reflector (metals) and dielectric materials. These can be made of three layers (as shown in Figure 10), or a second type, which has a core of reflective material which in turn is covered by a symmetrical system of weakly refractive and partially reflective layers, for a total of five layers (shown in Figure 11).
In this second type of OVP, the center "inner reflector" layer is usually aluminum, and the "low refractive" layers can be either SiO2 or MgF2 or Fe2O3; and the "partially reflective" outer layers can be Al, Cr, MoS2, Fe2O3.10 None of these layers has any color of its own, but when they are put into close contact with each other, they give rise to brilliant colors because of the components selective destructive and constructive interference as light reflects off the interfaces.11
OVPs' entire color play cannot be seen with the usual flop movement that is characteristics of metallic pigments. Extreme illumination and observation positions, face angle and grazing angle, are necessary to see all the color shades of the pigments.
Changing the thickness of the interference layers changes the observed colors. The usual shifts are magenta to green; green to blue; and gold to green. Usually they are printed with intaglio presses with an engraving depth of 50-70 microns with a regular pattern.12 The pigments are usually 1 micron in total thickness and the tolerances are very tightly controlled (see Figure 12).13
Some examples of OVP prints can be seen in the currency samples either in your pocket (look for the new $100, $50, $20, $10 and $5 bills), or as shown in Figure 13.

Photochromic Colorants
There are various organic photochrome dye molecules, such as: triarylmethanes, stilbenes, azastilbenes, nitrones, fulgides, spiropyrans, naphtho pyrans and spiro-oxazines. Almost all of these molecules have similar mechanisms of photochromism. As an example, the oxazines mechanism will be explained.The colorless form of the oxazines contains a spiro carbon atom. This carbon atom is SP3 hybridized and serves to separate the molecule into two halves. Each half is comprised of benzenoid heterocyclics whose pi systems are orthogonal and are not conjugated across the spirocarbon atom. Because the localized pi systems are separated by this SP3 hybridized carbon, all absorptions are in the UV part of the spectrum, not in the visible. Hence, the molecule appears colorless in its unactivated form.
Upon exposure to UV radiation, the bond between the spiro and the oxygen of the oxazine ruptures, and the molecule undergoes a geometric rearrangement. In this new "ring opened" form, the pi systems are joined and conjugated along the entire molecule, which is virtually planar. This lowers the energy of the transition, and thus, the molecule absorbs at longer wavelengths, i.e., in the visible spectrum. For the blue photochromics, this absorption is around 610 nanometers. The absorption of red photons from white light leaves a remaining color that is blue. This is the same explanation for why the sky is blue.
On removal of the UV source, the molecule relaxes to its original geometric conformation -- the closed spiro form with the carbon oxygen bond reforming. The net observation is a return to the colorless state. This bond breaking and reforming process combines to form a cycle that goes from the closed colorless spiro form to the open colored merocyanine form and back. All these photochromic dyes, whether oxazine or naphthopyran, function in much the same way.14
Four basic colors are usually available: a violet, a blue, a yellow and a red. These colorants can be mixed like process colors to make some new colors such a by mixing a blue and a yellow to get a green, etc. These colorants can easily be mixed in most common ink solvents, but sometimes it is better to use the "pigmentary" form that is manufactured by microencapsulating the dyes in a carrier resin. The particle size distribution is usually between 1-6 microns, which lends itself to use with most printing systems if proper precautions are taken. Some of these colorants have excellent weather ability and light fastness, and some others have quite poor light and chemical fastnesses. It is best to discuss with the suppliers prior to using them

Thermochromic Colorants
Thermochromic colorants can be both inorganic and organic. Amongst the inorganic chemicals that have thermochromism are cobalt chloride, which changes from a faint pink to a blue at 35 deg C; and nickel sulfate, which is green at room temperature but becomes yellow at 155 deg C.The thermochromic mechanisms of the organic molecules are different than the ones for inorganics. Most of these molecules can be custom manufactured to work in different temperature ranges. For example, one manufacturer offers nine different colors and temperature ranges from -15 deg C to 70 deg C.
These colorants are best used in either an emulsion form or in a quasi pigmentary form (microencapsulated) and again their particle sizes are between 1-6 microns, and can be used by most common printing methods: these range from screen inks for T-shirts or coffee mugs to offset litho for security applications. These dyes have are sensitive to light and some chemicals and it is advisable to discuss with the suppliers prior to using them.
Conclusion
All these colorants have unique characteristics that make them useful in printing inks. Some have been with us for a very long time, others are quite recent innovations. Everyday there are new uses that are being created. Learn how to use them properly, innovate with them and you will advance the art of ink making and printing.This article is based on a presentation given at the 2001 NPIRI Summer Course Advanced Topics: Raw Materials & Additives for Specialty Inks & Coatings.
For more information on special-effect pigments, contact A. Nurhan Becidyan, United Mineral & Chemical Corp., 800-777-0505 or e-mail anbecidyan@umccorp.com.
References
1 Becidyan, A.N. United Mineral & Chemical Corp., Lyndhurst, NJ, Luminescent Pigments in Security Applications, COLOR Research and Application, Vol. 20, Number 2, April 1995.2 Private Correspondence with Streitel, Steven, Day Glo Corp., Cleveland, OH, (Copyright 1990-2001© Steven Streitel).
3 Copyright ©1995-1998 by Amethyst Galleries, Inc. from http://mineral.galleries.com/agi.htm.
4 Fundamentals of Luminescence, Shionoya, S. and Yen, W.M., Editors, Phosphor Handbook, CRC Press, P. 61, 1998.
5 Springsteen, A.W., Ph.D. Labsphere Inc., North Sutton, NH, Fluorescence & Color, a monograph published by Labsphere Inc., North Sutton, NH.
6 Byler, William H., Luminescent Pigments, Inorganic, in Handbook of Pigments, John Wiley & Sons, I-I-b, 1977, pp.905-923.
7 LumiNova[r] is a Registered Trademark of Nemoto & Co., Ltd. of Tokyo, Japan.
8 OVP[r] is a Registered Trademark of Flex Products Inc., Santa Rosa, CA.
9 Schmid, Raimund, et al, BASF AG, Ludwigshafen, Germany, from a paper presented at the 4th NŸrnberg Congress" (NŸrnberg, 7-9 April, 1997), which was jointly organized by the Paint Research Association, Teddington, UK, and Vincentz Verlag, Hannover, German.
10 Iden, RŸdiger, et al, BASF AG, Ludwigshafen, Germany New Challenges in the Ways of Making Color, Modern Paint and Coatings, April 1999, pp 32-40.
11 Parker, Barbara, Flex Products Inc., Santa Rosa, CA, Using New High performance Color Shift Pigments to Create Dramatic Color Effects, from a paper presented at RETEC 1998.
12 Bretler, Haim, SICPA S.A., Switzerland, Thin Film devices in Security Printing Inks, paper given at Conference on Optical Security, Los Angeles 1990.
13 Figure courtesy of Flex Products Inc., Santa Rosa CA. Copyright 1995-2001[C] Flex Products Inc., Santa Rosa CA
14 PPG's Photosol[r] Photochromic Dyes, Copyright 1996-2001[c] PPG Industries Inc. from http://www.ppg.com/_private/ FrameResult.asp?f=/chm_optical/psol/default.htm

Report Abusive Comment