

Polycarbonate is used in many automotive applications due to its excellent properties of impact resistance, high heat distortion temperature and clarity. When used to replace glass or steel, polycarbonate also brings the added benefit of design freedom deriving from injection molding and low part weight. These benefits come at the cost of poor abrasion resistance, weatherability and chemical resistance. To meet application requirements for the first polycarbonate headlamps in the mid 1980s, thermally cured silicone abrasion-resistant hardcoats were developed to augment performance. Coated headlamps were able to meet the Department of Transportation’s minimum requirement of three years’ Florida and Arizona weatherability, as called out in SAE J576 JUL91 (see Table 1).1 In 1989, GE Silicones introduced AS4000 hardcoat and primer SHP401. This system (referred to as AS4000) extended coating delamination in Florida and Arizona testing well beyond the SAE minimum requirements, making it one of the leading coatings in use for headlamps even today, over a decade later. The success of AS4000 led to its expansion into other applications such as sunroofs and pillar posts, which was begun by Lexamar Corp. in 1989.
Current Thermal Hardcoat Capabilities
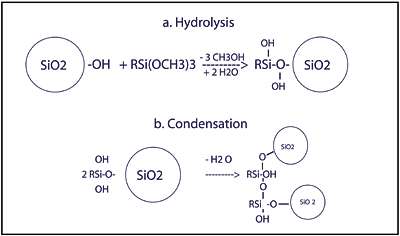
The abrasion resistance and ultraviolet (UV) light protection of the AS4000/SHP401 system derive from the AS4000 topcoat. This type of topcoat, is prepared by the hydrolysis of alkoxysilanes in the presence of water and colloidal silica (see Figure 1), and can contain an alkoxylsilylated UV absorber2for protection of the polycarbonate. AS4000 is offered at 20% nonvolatile content in aqueous alcoholic solvents.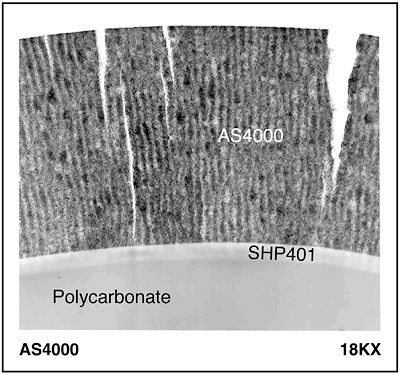

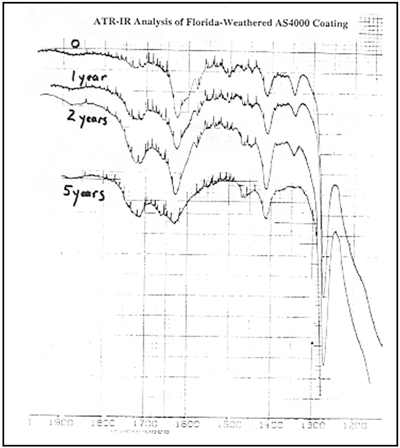


Improvements in UV Absorber Technology
Pickett followed up on GE UVA#1 degradation data by screening commercially available UV absorbers. Coatings of various proprietary and commercially available UV absorbers dispersed in typical commercial binders were prepared. The change in absorbance at 340 nm was measured as a function of exposure in xenon arc. What he observed was that the UV absorber performance was dependent on the matrix in which it was dispersed (see Table 3).5Two new types of proprietary UV absorbers demonstrated outstanding stability. We chose to look further at GE UVA #2 for a new acrylic primer and GE UVA #3 for a new silicone topcoat, both with the potential for five times the stability of GEUVA #1.Product and Process Design
Assessing Needs
The first part of the new product design was to determine what properties were Crucial to Quality (CTQs). Six-sigma based customer needs mapping revealed that a 10-year weatherable coating was desirable. Features of conventional thermal cure hardcoats that could not be lost in the new development were abrasion resistance, transparency and chemical resistance. In addition, the unspoken CTQs — those expected by the customer as a given — were properties such as ease of application and retained impact resistance.

Setting specifications
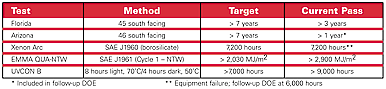
At the next level in product design, the concept of 10-year weatherability was broken down into measurable responses. The customer defined 10-year weatherability as “no more than 5% field failures of parts in service on a vehicle at 10 years.” To measure this CTQ on test panels effectively, a measurable target of a successful seven-year Florida and Arizona exposure was mutually agreed upon. Accelerated weathering targets equivalent to seven years’ outdoor exposure were also set, based on our experience with thermal hardcoats and average UV dosages at 340nm (see Table 4).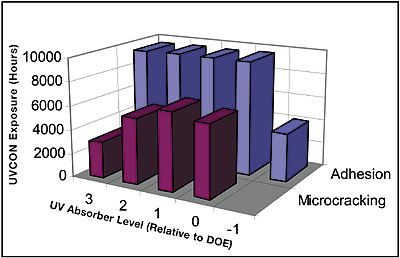
Screening Experiments
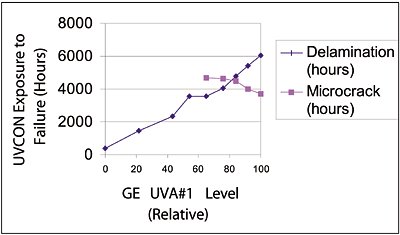
The new hardcoat technology was based on the strengths of AS4000/SHP401. By starting out with excellent adhesion, the objective became one of retaining that level of adhesion during a lifetime of outdoor exposure. The mechanism of adhesion failure for thermal hardcoats is the degradation of the polycarbonate surface. When this layer degrades, it turns yellow. The degraded, yellow polycarbonate gives a weaker interface, leading first to scribed adhesion loss and finally, to delamination. The novel approach used in developing this hardcoat system was to add enough of the new, highly stable UV absorbers to their respective primer and topcoat matrices to achieve the desired UV protection. The UV absorber level in the primer was adjusted with GE UVA #2 and a ladder experiment was designed around the level of GE UVA #3 in the topcoat to determine what additional UV absorbance would be required. A series of coatings with increasing UV absorber level in the topcoat was prepared and coated on Lexan LS2 polycarbonate. Coated pieces were subsequently exposed in a UVCON. A critical fact observed from this experiment was that having no UV absorber in the topcoat (coded –1 in our subsequent DOE) led to premature adhesion loss and eventually to delamination. At the high end of UV absorber level, (coded +3 relative to our subsequent DOE) we encountered premature microcracking (see Figure 7). The tendency to microcrack with high levels of UV absorber had been observed earlier with experimental coatings (see Figure 3), so a similar trend here was not unexpected. The other result from the ladder experiment was the preliminary confirmation that the matrix chosen was stable enough to deliver unusually good UVCON results relative to the >3,000 hours afforded by coatings containing GE UVA #1. It would be necessary in the full DOE to confirm this benefit through other accelerated weathering methods and outdoor weathering.

Design of Experiments (DOE)
The next step in the development was to look critically at the components of the ladder study to select candidate factors in addition to UV absorber that might intuitively have an influence on weatherability. Again, drawing upon our experience in hardcoats, five product factors were varied. A sixth factor, the effect of duration of cure at 130°C, was also added. Oven times were chosen at 30 and 90 minutes, including approximately five minutes of heat-up time. To make the scope of this DOE more manageable, a 2(6-2) fractional design, giving 16 runs, was set up. Two center point replicates were added to determine curvature. The whole DOE was then replicated at a slightly greater top coat thickness. The total number of runs was 36). After the DOE coatings were made, they were coated and cured on clear, Lexan 9030 polycarbonate panels. Panels were then cut into smaller test pieces if required for accelerated and outdoor weathering testing established in Table 4.
The three-year Florida results are shown in Table 6 relative to the predictions made by accelerated data. The correlation with failed coatings at this point in time is good, although the exact time of failure does vary somewhat. Failures are confined to most of the “D” group from the DOE and coating “C4” which correlate with factor “b” and possibly factor “a” being at the “+1 coded level.
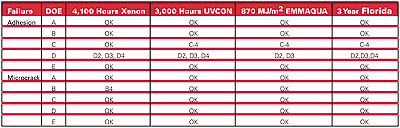

The data for adhesion loss in EMMAQUA at 2,900 MJ/m2 exposure (the equivalent of 10 years’ Florida) give a simplified coded transfer function: EMMAQUA adhesion failure (in MJ/m2) = 2376 – 498a – 299b + 517c – 281d – 335f – 281 af + 169 x coating thickness. This equation says that the coating lasting the longest in EMMAQUA will have low levels of factors a and b, and a high level of factor c as predicted, with d and f plus an interaction term also being significant. The best three coatings from the DOE are A2, C3 and D1. At this time, work continues toward determining the most robust product and process meeting all CTQs.
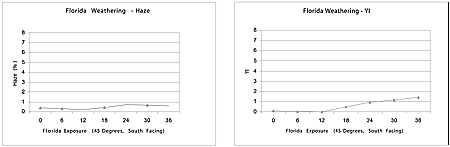
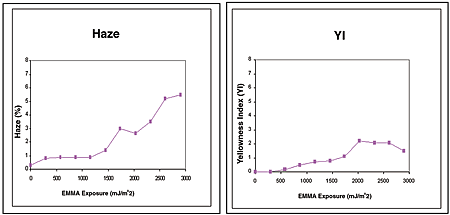
The underlining impetus in developing the new coating to raise the bar toward 10-year weatherability. In comparing the data to that for the current SAE J576 specification, it is apparent that this new coating excels at both microcracking and adhesion, with accelerated data that is at least twice as good as the specification. The accelerated weathering has progressed to the point where EMMAQUA has reached the 10-year Florida equivalent in weathering (see Table 8).

Coatings on Black Polycarbonate
While the work described above was in the early stages of evaluation, a similar DOE was initiated on black polycarbonate. The accelerated weathering data showed the same trends for factors “a”, “b” and “c” as in the first DOE and the best coatings still look good after three years’ Arizona exposure (see Figure 14). The three year Florida panels are expected to be returned shortly. A follow-up DOE involving the processing of coating A2 on black polycarbonate is still in test. We are currently focusing on optimizing manufacturing parameters during scale-up and application on clear and black polycarbonate, based on data from these several product and process DOEs. Accelerated and outdoor weathering for those studies will be continued until failure.
Applications
LexaMar a division of Decoma International, is a leading supplier of hardcoated automotive products and expects to supply more than 6 million parts for automotive use this year. Started in 1985, LexaMar specializes in the design, manufacture and assembly of hardcoated injection molded exterior parts for automotive use. The company started using GE Silicones AS4000 hardcoat in 1989 and presently the facility is in the process of building a state of the art process to apply the new GE Silicones product, AS4700. This product derives from the above described work and is expected to greatly extend part weatherability, approaching the 10-year milestone. The current plan calls for start-up of the new hardcoat system by the end of 2001.
Summary
GE Silicones has developed a new hardcoat for polycarbonate that raises the protection of polycarbonate to a new level. New, highly stable UV absorbers were combined with improvements in existing coating chemistries using DOEs to survey the product and process design spaces. Accelerated weathering tests were conducted using UVCON-B, xenon arc and EMMAQUA to predict the suitability of these new coatings. Outdoor exposure testing shows data at three years in Florida compares favorably with the accelerated weathering predictions. The critical factors for weatherability and abrasion resistance have been identified and an optimized product has been selected. Processing optimization on clear and black polycarbonate is still under investigation.The International Coatings for Plastics Symposium is an annual event sponsored by PCI Magazine. For more information, contact Harper Henderson at 248/244.6478, visit www.pcimag.com, or e-mail harperh@bnp.com.
For more information on hardcoats, contact George Medford, General Electric Silicones LLC, 260 Hudson River Road, Waterford, NY 12188.






Report Abusive Comment