
The cure speed and film properties can be tailored to the application by varying the hardness (Tg) and functionality (EW or OH number) of the acrylic polyol; the isocyanate and solvents used; and by the addition of amine, tin or zirconium catalysts and activators. Heat can also be applied to accelerate the cure. Generally, the faster the cure, the shorter the pot life and the higher the VOCs. This is a significant limitation of 2K urethane coatings since one has to trade productivity for compliance, cost and ease of use.

UV-curable formulations typically contain an acrylated oligomer based on a polyether, polyester or epoxy resin. These oligomers are more difficult to prepare than the acrylic polyols used in 2K urethanes, which contributes to the high cost of UV-curable formulations. It is even more difficult to prepare acrylated urethane oligomers based on acrylic polyols because their high functionality often leads to gelling or high viscosities. We believe this has contributed to the slow growth of UV-curable coatings in applications where excellent UV stability is required, such as automotive clearcoats. Other limitations of UV-curable coatings include the absence of a dark cure mechanism, difficulty in spraying and curing 3D parts, and poor adhesion and flexibility due to shrinkage during the curing process.
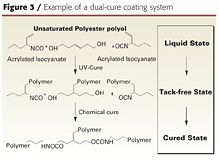
Several companies have recently reported on dual-cure coatings that combine the benefits of UV and 2K urethane systems and overcome some of their limitations (Figure 31). These coatings are based on acrylated polyisocyanates and unsaturated polyester polyols. The UV-curing step is used to obtain tack-free coatings so the parts can be handled, buffed or sanded immediately after UV cure. Another benefit of achieving an instantaneous, tack-free state is that the coating appearance is improved. This is because fewer airborne impurities have time to contact the wet film and cause defects.
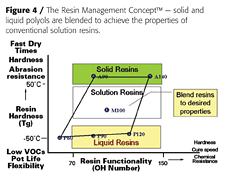
We have recently developed a family of acrylic polyols based on allylic alcohols.2 These new acrylic polyols are unique in that they are solvent-free, solid and liquid resins with very low solution viscosities and superior functionality compared to conventional acrylic polyols. These polyols are also designed to be blended together to achieve coating properties for a wide range of applications and technologies, including high solids, UV- and moisture-curable, and powder coatings (Figure 4).
The following paragraphs describe the preparation and properties of new acrylic urethane coatings for weatherable applications. These include 1K/UV-curable systems based on novel acrylated urethane acrylic oligomers, and 2K/UV-curable systems based on novel acrylic polyols and their partially acrylated urethane derivatives. These coating systems offer instant cure and superior properties compared to conventional UV-curable systems. These systems may allow UV-cure technologies to be used in the most demanding applications including automotive topcoats.
Results and Discussion
1K Acrylic Urethane UV-Cured Coatings
Most acrylated oligomers are based on polyether, polyester and epoxy resins. This is because the functionality of these condensation resins can be precisely controlled to 2 or 3 to prevent gelling and keep viscosities low (Figure 5). The weatherability of these resins is usually inferior to acrylics.
Acrylic polyols, on the other hand, are produced by free-radical polymerization and are usually supplied in solution. So the solvent has to be removed to produce solvent-free UV-curable coatings. More importantly, the functionality of acrylic polyols cannot be easily controlled. Consequently, the traditional two-step synthesis shown above typically leads to highly crosslinked, viscous, or even gelled products that are not suitable for coating applications. This is why there are few, if any, acrylated urethane acrylics on the market today. Nonetheless, we were able to prepare low-viscosity urethane acrylates based on the new liquid acrylic polyols.

Synthesis of Acrylated Urethane-Acrylic Oligomers
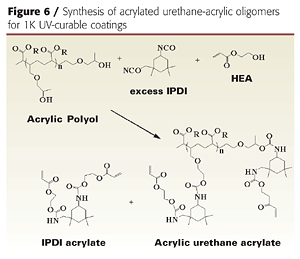
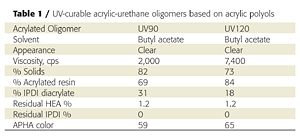
Initial attempts to prepare acrylic-based prepolymers with HDI (hexamethylene diisocyanate), TDI (toluene diisocyanate) or IPDI (isophorone diisocyanate) led to gelling. IPDI was ultimately chosen for the unequal reactivity of the primary and secondary isocyanate groups, which helps control crosslinking and prevents gelling.3 By modifying the process and reagents slightly, we were able to produce gel-free, low-viscosity, low-color acrylated urethane-acrylic oligomers (Table 1). The resins also contained 20-30% of IPDI diacrylate, which is produced when excess IPDI reacts with hydroxyethylacrylate (HEA).
The liquid acrylic polyol was first treated with excess IPDI to form an isocyanate pre-polymer, which was then capped with HEA. Excess IPDI is required to prevent gel formation when preparing the isocyanate prepolymer, because the hydroxyl functionality of the acrylic polyol is greater than 5. The resulting product is a mixture of acrylated acrylic-urethane oligomer and acrylated IPDI reactive diluent.
The butyl acetate solvent we used to reduce viscosity can be replaced with acrylate monomers such as IBOA (isobornyl acrylate) to produce solvent-free and VOC-free formulations, albeit with much higher viscosity. Alternatively, low photochemical reactivity solvents such as acetone can be used to produce formulations that are VOC-free (exempt status in the U.S.) and have limited ozone-forming potential.
Acetone is an excellent viscosity reducer for these resins and a good choice for these systems. It is inexpensive and evaporates quickly, which allows the coating to be sprayed without a lengthy flash-off time. It is important, however to allow the solvent to flash off to prevent the formation of pinholes and solvent popping during the UV cure. The final coating hardness is also improved if the solvent is allowed to escape before the coating is UV cured.
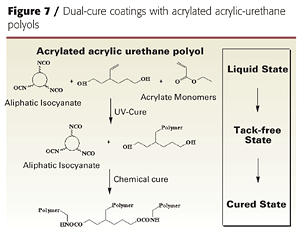
The same chemistry was used to produce low-viscosity, acrylated urethane-acrylic resins with residual OH functionality. The residual OH functionality provides a crosslinking site for dark or thermal cure with conventional isocyanates or melamine crosslinkers (Figure 7).
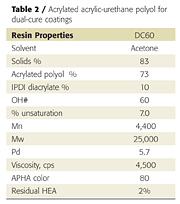
However, instead of using excess IPDI, the acrylic polyol was first treated with two equivalents of isocyanate in the presence of tin catalyst to reduce the hydroxyl functionality to 3-4 per polymer chain, and then treated with two equivalents hydroxyethylacrylate (HEA). The resulting resin had an average of 2 acrylate and 3.6 hydroxyl groups per polymer chain, making it capable of both UV and chemical crosslinking reactions (Table 2). This resin can be used in dual-cure coatings diluted with acrylate monomers but does not, by itself, give tack-free coatings after UV cure.
Coating Formulations and Properties
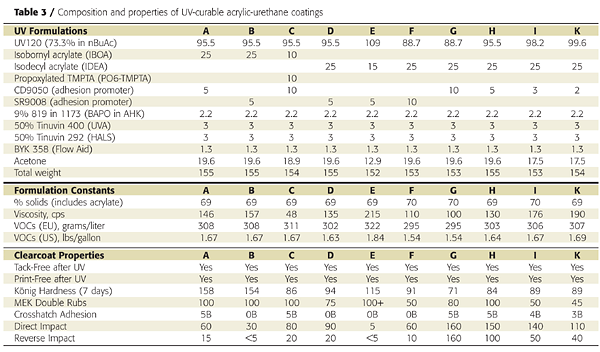
Our selection of diluents and solvents was dictated by the following requirements:- High UV reactivity (monomers);
- Low shrinkage (monomers);
- Good balance of hardness and flexibility (monomers);
- High UV stability after polymerization (monomers);
- Good viscosity reduction (monomers and solvents);
- Low toxicity and irritancy (monomers and solvents);
- Fast evaporation (solvents); and
- Non-HAP and VOC exempt (solvents).
We chose to add solvent to these formulations because we wanted a sprayable system with good sag resistance and minimal shrinkage during UV cure. Because of the relatively poor solvency of acrylate monomers, we would have had to use large amounts of monomers to reduce the system to spray viscosity, thereby increasing the chance of shrinkage and poor adhesion.
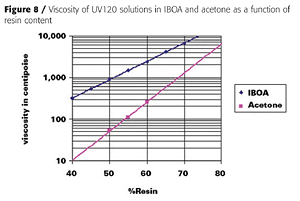
Furthermore, a sprayable coating system prepared only with acrylate monomer diluents would be much more likely to sag when applied to vertical surfaces. We chose acetone as the solvent because of its rapid evaporation rate, excellent solvency, and non-HAP and VOC-exempt status in the U.S. Although not VOC-exempt in Europe, acetone has low ozone-forming potential and should have minimal impact on tropospheric ozone compared to other, more reactive solvents. Figure 8 illustrates that acetone is a much better solvent than IBOA for UV120.
1K, UV-Cured Acrylic-Urethane Coatings
Clear coatings were prepared using the fully acrylated urethane-acrylic resin and various acrylate monomers and adhesion promoters. The coatings were applied to primed metal panels, allowed to flash off, and irradiated 30 seconds (12 passes at 2.5 seconds per pass) with a D-bulb (20.5 Joules/cm2). All coatings were tack- and print-free after irradiation. Conversion of the acrylate unsaturation was measured by Raman spectroscopy and was typically 80% after the first pass and above 90% after the 12th.
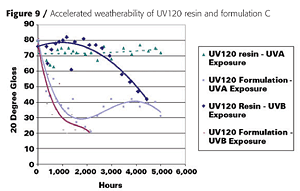
Film properties were highly dependent on the monomer diluents but were comparable to conventional 2K urethane coatings. The VOC (U.S. definition) content was below 1.7 lb/gallon, which is comparable to a waterborne coating. Because Europe does not consider acetone VOC-exempt, the VOC content for Europe is around 300 grams per liter. After 5,000 hours of UV-A and -B exposure, the base UV120 resin showed good to excellent gloss and color retention (Figure 9).
UV screeners improved the weatherability of both coatings yet did not interfere with the UV-curing process. This is because the BAPO photoinitiator absorbs in the near-UV/visible range and the HALS and UV screeners do not.
2K, Dual-Cure Coatings
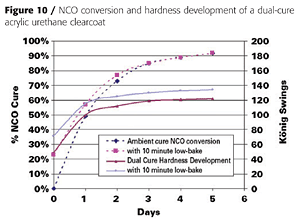
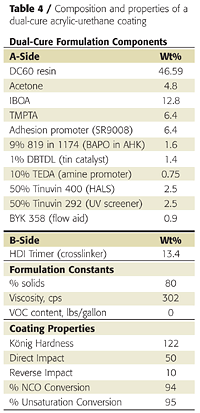
Clear coatings were also prepared using the partially acrylated polyol DC60. Unlike the fully acrylated version, DC60 does not yield a tack-free coating by itself when exposed to UV light. It must be dissolved in acrylate monomers. We again used IBOA as the monofunctional acrylate and TMPTA as the multifunctional acrylate. We also used a different adhesion promoter (SR9008 from Sartomer) and added a tin catalyst and amine synergist (triethylenediamine or TEDA) to accelerate the dark cure reaction between the OH groups and the isocyanate crosslinker (Table 4).Because the polyol and isocyanate crosslinker (HDI trimer) react under ambient conditions, they must be mixed shortly before application. This is typical of 2K urethane coatings. This reaction also provides a dark-cure mechanism that is not found in conventional UV-cured systems. The NCO-OH reaction, however, is too slow to provide a tack-free state instantaneously. However, the UV-cure component provides instantaneous hardness development, as illustrated in Figure 10.
Coating hardness was 49 König swings immediately after UV cure, and the coatings was tack- and print-free. The NCO-OH reaction continued for another week, during which the coating hardness increased to 120 swings. Heating the coating to 60 °C for 10 minutes after UV cure accelerated the NCO-OH reaction and hardness development and resulted in a slightly harder final film.
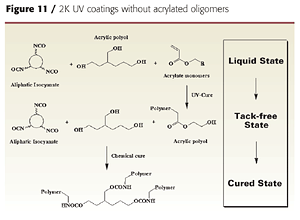
2K UV Coatings Without Acrylated Oligomers
It is also possible to develop dual-cure coatings using conventional 2K urethane polyol ingredients and acrylate monomers as diluents. This may potentially reduce the cost of dual-cure formulations by eliminating the need for acrylated oligomers. The acrylate monomers help reduce the viscosity of the polyol and isocyanate crosslinker and are polymerized with UV light to achieve a solid state. Adding a hydroxy-functional acrylate monomer (such as HEA) provides a crosslinking mechanism between the UV-generated acrylic resin and the isocyanate crosslinker (Figure 11).The final coating properties and cure speed are controlled by the formulation components, their relative amounts and their order of addition. Since neither the polyol nor the isocyanate crosslinker are involved in the UV-curing reaction, the acrylate monomers, acrylic polyol and isocyanate crosslinker must be carefully selected so that a tack-free state is achieved after UV cure.
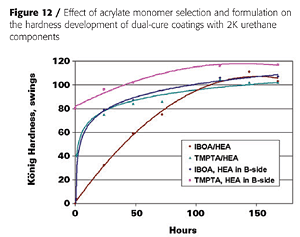
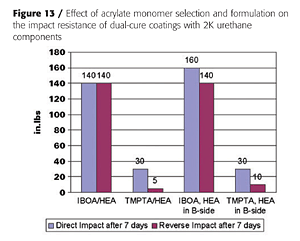
With liquid acrylic polyols as the base polyol and HDI-trimer as the isocyanate crosslinker, mono-functional acrylates such as IBOA (isobornyl acrylate) do not yield a tack-free state after UV cure, unless the HEA is pre-mixed with the isocyanate, as is illustrated in Figure 12. This, presumably, generates an acrylated polyisocyanate that can now be involved in the UV-curing process. Some tri-functional TMPTA (timethylolpropane triacrylate) is usually required to build enough molecular weight. However, the impact properties of the final coatings suffer as is expected from the higher crosslink density (Figure 13).
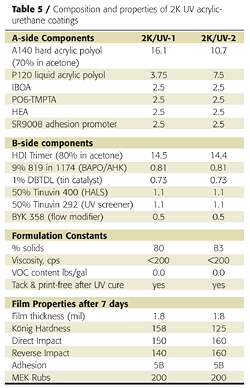
Replacing a portion of the liquid polyol with a high-Tg polyol was also effective in achieving a tack-free state immediately after UV cure. This approach is preferred over the use of TMPTA because the final coating has better impact resistance and flexibility. Zero-VOC formulations sprayable at 80% solids and above were achieved using acetone and acrylate monomers (Table 5). Film appearance and physical properties were also excellent.
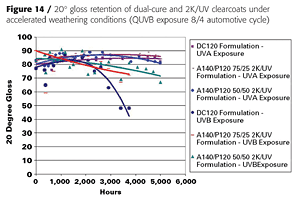
The cured dual-cure and 2K/UV clearcoats were also subjected to accelerated weathering tests under QUVA and QUVB conditions. After 5000 hours of exposure, all coatings have retained over 90% of their gloss, even under harsh QUVB conditions (automotive cycle) (Figure 14).
Conclusions
Low-VOC, sprayable acrylic-urethane clearcoats have been developed for weatherable applications. One-component, UV-curable coatings were prepared using novel acrylated urethane-acrylic resins. Two-component coating systems were developed using a partially acrylated urethane acrylic polyol and conventional 2K urethane components, diluted in acrylate monomers and acetone.These coatings have excellent appearance, physical properties and weatherability, suggesting that acrylic-urethane UV-curing clearcoat technology could potentially be applied to automotive OEM and refinish applications, as well as wood and plastic parts. The 2K/UV formulations are particularly interesting, in that they offer the instantaneous cure and productivity of UV-curable coatings while maintaining the physical properties, weatherability and low-cost characteristics of conventional 2K urethane systems.
Tinuvin® 400 and 292 are products of Ciba Specialty Chemicals. BYK® 358 is a product of BYK Chemie.
Resin Management Concept is a registered trademark of Lyondell Chemical Company.
AcknowledgementsThe authors wish to thank Ann Good, Bob Good, Dave Pangburn and Mark Smithson for expert technical assistance, and Steve Harris for helpful discussions.
For more information, contact the company at 3801 West Chester Pike Newtown Square, PA 19073; phone 610/359.2000; e-mail acryflow@lyondell.com; or visit www.lyondell.com.
References
1 Fischer, W.; Weikard, J.; and Meier-Westhues, U. "Dual Cure; Combination of Superior Properties", Radtech Report, November/December Issue, (2001).2 Guo, S.-H.; Wang, W.; Harris, S. H.; Patel, S.; Junker, L. J.; Blackwell, R. S.; Fadakar, F.; and Pourreau, D. B. "New Acrylic Polyols for Low-VOC Coatings", PCI Magazine, June Issue, (2002).
3 Lomoelder, R.; Plogmann, F.; and Speier, P. "The Selectivity of Isophorone Diisocyanate in the Urethane Reaction. Influence of Temperature, Catalyst, and Reaction Partners", PaintIndia, November Issue, p.31, (1998).
This paper was presented at the 7th Nurnberg Congress, European Coatings Show, April 2003, Nürnberg, Germany.




Report Abusive Comment